Suspected Copper Chelator Binds not just Copper but Copper-Protein Trimer
Complexes
summary written by Raven Hanna
Cells need copper to function, but too much copper can be toxic, leading to liver damage and neurological problems, as happens in disorders such as Wilson disease. The inorganic small molecule tetrathiomolybdate (TM), assumed to be a copper chelator, is commonly used to treat Wilson disease. TM may also be an effective treatment of some cancers by starving the cancer cells of the copper they need to grow. Despite its common use, its molecular mechanism was unknown.
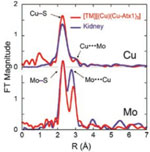
A team of researchers led by Profs. Thomas O'Halloran and Alfonso Mondragón of Northwestern University and Jim Penner-Hahn of University of Michigan used SSRL Beam Line 9-3 to solve the solution electronic and geometric structure (XAS) and SBC-CAT and IMCA-CAT at APS to determine the three-dimensional crystal structure of TM bound to copper and its chaperone protein. They were surprised to find that TM does not simply sequester copper from Atx1, the copper chaperone, but binds to a copper-protein trimer complex in a nest-like structure, completely disrupting the copper transport system. The TM binds to the copper atoms that are bound to the cysteine sulfurs of chaperone proteins.
Understanding the way TM can shut down the copper ferrying system could lead to
treatments for some cancers, since growing cancers require a supply of copper.
TM may also be useful for treating other diseases, such as familial amyotrophic
lateral sclerosis (ALS), Parkinson's disease, multiple sclerosis, Alzheimer's
disease, and some disorders associated type II diabetes. The research was
published in the January 15 issue of Science.
To learn more about this research see the full Scientific Highlight
H. M. Alvarez, Y. Yue, C. D. Robinson, M. A. Canalizo-Hernández, R. A. Marvin, R. A. Kelly, A. Mondragón, J. E. Penner-Hahn and T. V. O'Halloran, "Tetrathiomolybdate Inhibits Copper Trafficking Proteins through Metal Cluster Formation", Science 327, 331 (2010) doi: 10.1126/science.1179907