

The copper sequestering drug tetrathiomolybdate (TM) has been shown in studies
to be effective in the treatment of Wilson disease, a disease caused by an
overload of copper, and certain metastatic cancers. That much is known. Very
little, however, is known about how the drug works at the molecular level.
A new study led by Northwestern University researchers now has provided an
invaluable clue: the three-dimensional structure of TM bound to copper-loaded
metallochaperones. The drug sequesters the chaperone and its bound copper,
preventing both from carrying out their normal functions in the cell. For
patients with Wilson disease and certain cancers whose initial growth is helped
by copper-dependent angiogenesis, this is very promising.
This knowledge opens the door to the development of new classes of
pharmaceutical agents based on metal trafficking pathways, as well as the
further development of more efficient TM-based drugs. The study was first
published in Science Express in November.
"Essential metals are at the center of many emerging problems in health,
medicine and the environment, and this work opens the door to new biological
experiments," said Thomas V. O'Halloran, the study's senior author and the
Charles E. and Emma H. Morrison Professor of Chemistry in the Weinberg College
of Arts and Sciences at Northwestern. He and geneticist Valeria Culotta of
Johns Hopkins University discovered the first copper chaperone function in
1997.
O'Halloran and his research team studied the copper chaperone protein Atx1,
which provides a good model of copper metabolism in animal cells. "We wondered
what the drug tetrathiomolybdate did to copper chaperones — proteins
charged with safely ferrying copper within the cell — and what we found
was most amazing," O'Halloran said. "The drug brings three copper chaperones
into close quarters, weaving them together through an intricate metal-sulfur
cluster in a manner that essentially shuts down the copper ferrying system."
The nest-shaped structure of the metal-sulfur cluster discovered by the
researchers was completely unanticipated.
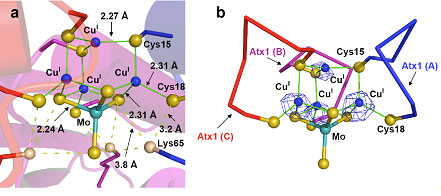
Figure. 1:
Structure of the nest-shaped [S6Cu4MoS4]
cluster in the [TM][(Cu)(Cu-Atx1)3] trimer complex. (A) Structure of the
[S6Cu4MoS4] cluster with average interatomic
distances. (B) Cu anomalous peaks in the final model of the
[S6Cu4MoS4] cluster (blue mesh of the
anomalous difference Fourier map are contoured at 10.0 s level)
Alfonso Mondragón, professor of biochemistry, molecular biology and cell
biology in the Weinberg College of Arts and Sciences, and graduate student Yi
Xue, both co-authors of the paper, solved the three-dimensional crystal
structure using protein X-ray crystallography. This is the first example of a
copper-sulfide-molybdenum metal cluster protein.
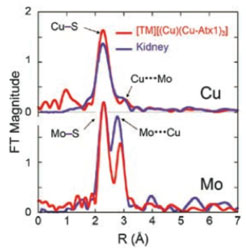
Figure 2:
Cu and Mo K-edge extended x-ray absorption fine structure (EXAFS) Fourier
transforms phase- shift overlay (experimental data) for
[TM][(Cu)(Cu-Atx1)3],
and a kidney sample extracted from LPP rats treated with TM.
Based on the structure and additional experiments, the scientists propose that
the drug inhibits the traffic of copper within the cell because of its ability
to sequester copper chaperones and their cargo in clusters, rendering the
copper inactive.
"We conclude that the biological activity of tetrathiomolybdate does not arise
from a simple copper sequestering action but through a disruption of key
protein-protein interactions important in human copper metabolism," Alvarez
said.
Inorganic elements, such as copper, zinc and iron, are vital to the healthy
functioning of all cells in living organisms. But they are high-maintenance
nutrients, and too much can be toxic, as is the case in Wilson disease, a
genetic disorder that prevents the body from getting rid of extra copper and
leads to liver and neurological problems.
Copper also is an important cofactor for tumor angiogenesis, the process of
growing new blood vessels to feed the tumor. Researchers believe this is why
tetrathiomolybdate has shown promise as an anti-cancer drug.
The chain of discovery that led to the use of tetrathiomolybdate as a
therapeutic agent began in the 1930s when cows grazing in certain types of
pastures in England developed neurological problems. This trouble was then
linked to other neurological problems with sheep grazing on certain soils in
Australia. It was found that molybdate, a non-toxic compound present in the
grass of these pastures, when consumed in excessive amounts by the ruminants,
led to copper deficiencies and neurological problems in the animals.
As copper overload disorders such as Wilson disease were discovered in humans,
physicians used molybdenum chemistry focusing on tetrathiomolybdate to lower
copper levels in the body. (Tetrathiomolybdate is an inorganic small molecule
first synthesized by J. J. Berzelius in 1826.)
Tetrathiomolybdate is the active pharmaceutical agent in a well-tolerated drug
that has shown activity for the treatment of Wilson disease and now is in phase
II clinical trials as an anti-cancer drug.
TM also has been examined in recent studies where copper dysregulation is
implicated in the pathogenesis of neurodegenerative diseases such as familial
amyotrophic lateral sclerosis (ALS), Parkinson's disease, multiple sclerosis
and Alzheimer's disease as well as primary pulmonary hypertension and left
ventricular hypertrophy associated with type II diabetes. Copper modulating
agents including TM have been shown to be active in animal models of these
diseases providing a rationale for advancing tetrathiomolybdates into clinical
evaluation in these areas.
Primary Citation
H. M. Alvarez, Y. Yue, C. D. Robinson, M. A. Canalizo-Hernández, R. A. Marvin,
R. A. Kelly, A. Mondragón, J. E. Penner-Hahn and T. V. Halloran,
"Tetrathiomolybdate Inhibits Copper Trafficking Proteins through Metal Cluster
Formation", Science 327, 331 (2010) doi: 10.1126/science.1179907
The National Institutes of Health supported the research.
"When we mixed TM together with copper chaperone proteins in a test tube, the
color of the solution changed from light orange to deep purple," said Hamsell
M. Alvarez, the paper's first author and a former doctoral student in
O'Halloran's lab, now with Merck & Co., Inc. "The sulfur atoms in the
tetrathiomolybdate bound to the copper atoms to form an open cluster that
bridged the chaperone proteins. In this manner, three copper proteins were
jammed onto one thiomolybdate." See Figure 1
In collaboration with Prof. J. Penner-Hahn, the researchers obtained Cu and Mo
K-edge EXAFS data on the active site of TM bound Atx1. Their data showed that
the copper was in the Cu(I) oxidation state, while the Mo EXAFS strongly
resembled that of tetrathiomolybdate Mo(VI). The EXAFS data were used to obtain
accurate near-neighbor bond distances from the Cu and Mo perspective (Figure
2).
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.