A tRNA Synthetase Domain Couples Aminoacylation and Editing Activities
summary written by Raven Hanna
The information encoded in genes is only useful if the decoder is accurate. The complicated process of translating information from nucleic acid to proteins incorporates systems that ensure accuracy and systems that edit inaccuracies. The ribosome has mechanisms to make certain that the codon on the messenger RNA matches the aniticodon on the transfer RNA before it adds the amino acid to the growing polypeptide chain. But it is just as critical that the tRNA is properly charged with the amino acid dictated by the genetic code. The synthetases that are responsible for placing amino acids on tRNAs have an editing feature that double-checks the match.
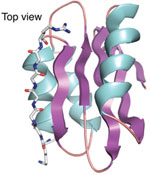
The synthetase that charges alanine tRNA has a structural domain that places the amino acid, a domain that removes the wrong amino acid, and a third domain with a previously unknown role. A group led by Professors Paul Schimmel and Xiang-Lei Yang at The Scripps Research Institute solved the crystal structure of this third domain of the alanine tRNA synthetase. They used SSRL Beam Line 11-1 to collect data and determined the structure at 1.85 angstroms using single anomalous diffraction. The structure revealed a known nucleic acid binding fold. This domain was shown through biochemical experiments to contact the elbow region of the tRNA.
The researchers propose that this domain promotes the coupling of the activities of the aminoacylation and the editing domains of alanine tRNA synthetase. Because phylogenic data shows that this domain has an ancient origin, it could have been the first bridge between these activities, ensuring that the tRNA bears the correct amino acid. This work was published in the August 7 issue of Science.
To learn more about this research see the full Scientific Highlight
Min Guo, Yeeting E. Chong, Kirk Beebe, Ryan Shapiro, Xiang-Lei Yang and Paul Schimmel "The C-Ala domain brings together editing and aminoacylation functions on one tRNA". Science, 325, 744-747 (2009).