Researchers Find a New Type of Copper-Protein Binding Site
summary written by Raven Hanna
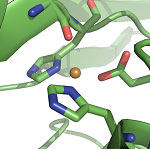
Copper is an essential ingredient for animal and plant life. Some proteins specifically bind copper for both structural and catalytic purposes. Up until now, mononuclear copper(II) ion binding sites fit into two categories, type 1 and type 2, defined by both their functional roles, structures, and the physical properties of the interactions.
Using a combination of spectroscopic and structure determination techniques and Cu K-edge XAS, Kyle M. Lancaster and Professors Harry B. Gray and John H. Richards of the California Institute of Technology found a novel copper-protein interaction and named the site "type zero." A specific mutation of a type 1 protein that uses copper for an electron transfer function results in the type 0 site. When initial analyses suggested that this copper site does not fit into either the type 1 or type 2 groups, the researchers solved high-resolution crystal structures of the protein. These revealed structural details that were unlike those found in either known type, in particular a distorted tetrahedral site with a very short copper-oxygen bond. To confirm their results and conclusions, they used SSRL Beam Line 7-3 for a copper-K edge and EXAFS study, in collaboration with Dr. Serena DeBeer George.
The researchers conclude that they have found a novel type of copper-protein interaction. They will continue to use SSRL to further characterize the properties of the type zero site and investigate its potential use as a catalyst in fuel cells. This research was published in the December 2009 issue of Nature Chemistry.
To learn more about this research see the full Scientific Highlight
Lancaster, K.M.; DeBeer George, S.; Yokoyama, K.; Richards, J.H.; Gray, H.B. Type Zero Copper Proteins, Nat. Chem. 2009, 1, 711-715.