

Nature adapts copper ions to a multitude of tasks, yet in doing so forces the
metal into only a few different electronic structures [1].
Mononuclear copper sites observed in native proteins either adopt the type 1
(T1) or type 2 (T2) electronic structure. T1 sites exhibit intense
charge-transfer absorption giving rise to their alternate title, blue copper
sites, due to highly covalent coordination by a thiol ligand donated by a
cysteine sidechain in their host proteins. This interaction has consequences
for the spectroscopic features of the protein, but more importantly gives rise
to dramatic enhancement of electron transfer activity. T2 sites on the other
hand resemble more closely aqueous copper(II) ions, and are found in catalytic
domains rather than electron transfer sites.
While investigating the factors that tune reduction potentials in the T2 C112D
variant of Pseudomonas aeruginosa azurin [2], Kyle M.
Lancaster, under the guidance of Harry B. Gray and John H. Richards at the
California Institute of Technology, discovered a copper(II) coordination mode
that did not easily fit either of the two classifications for a mononuclear
copper(II) site. The C112D/M121L azurin variant displayed one of the
fingerprinting features of T1 copper: namely, a narrow axial hyperfine
(A||) in
its EPR spectrum. However, the removal of C112 obviated any possibility for an
intense charge transfer band in the spectrum. Finally, electrochemical
measurements by Keiko Yokoyama demonstrated that this hard-ligand copper(II)
site could attain a copper(II/I) reduction potential typical for a T1 copper
protein. Not easily fitting into either of the classical regimes, the
investigators dubbed these species "type zero" copper proteins.
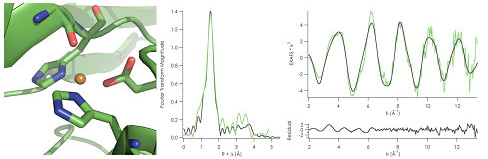
Left: Distorted tetrahedral active site of Copper(II) C112D/M121L azurin from
2.1 Å crystal structure. (PDBID: 3FPY) Right: Copper K-edge EXAFS of
C112D/M121L azurin fit starting from crystallographically-determined bond
distances.
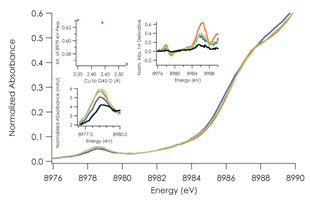
Figure 2.
Copper K-edge XANES of C112D/M121X (X= M, black; L, green; I, purple; F,
orange). Intensity gain at the 8979 eV preedge feature tracks with carbonyl
bond distance, and by extension tetrahedral distortion of the copper site.
Research at SSRL on "type zero" copper is ongoing at SSRL. EXAFS of the Cu(I)
"type zero" sites will be collected to complement crystallographic data,
allowing for assessment of the reorganization energy of these new electron
transfer sites. The investigators ultimately hope to incorporate these copper
sites into robust catalysts for fuel cell applications.
Primary Citation
Lancaster, K.M.; DeBeer George, S.; Yokoyama, K.; Richards, J.H.; Gray, H.B.
Type Zero Copper Proteins, Nat. Chem. 2009, 1, 711-715.
References
[1]
Malkin, R.; Malmström, B.G. The State and Function of Copper in Biological
Systems. Adv. Enzymol. 1970, 33, 177-243.
[2]
Lancaster, K.M.; Yokoyama, K.; Richards, J.H.; Winkler, J.R.; Gray, H.B. High
Potential C112D/M121X (X= M, E, H, L) Pseudomonas aeurignosa Azurins.
Inorg. Chem. 2009, 48, 1278-1280.
[3]
Gray, H.B.; Malmström, B.G.; Williams, R.J.P. Copper Coordination in Blue
Proteins. J. Biol. Inorg. Chem. 2000, 5, 551-559.
The investigators proceeded to grow crystals of the C112D/M121L azurin, as well
as the spectroscopically similar C112D/M121F and C112D/M121I variants.
High-resolution structures indicated two peculiarities in the "type zero"
copper structures. First, there was an unusually short copper to carbonyl
oxygen bond (2.35 - 2.55 Å). This interaction had until now been
observed with a distance of 2.6 Å at minimum. Secondly, the carboxylate
from D112 had reoriented into a decidedly monodentate interaction, with this
coordination mode presumed to be stabilized by hydrogen bonding from the amides
of N47 and F114. Previously implicated in effecting the decreased electron
transfer reorganization energy displayed by the native protein [3], the reestablishment of this hydrogen bond network was seen as
a suggestion that electron transfer activity had been enhanced relative to the
T2 C112D protein. This was subsequently confirmed by further electrochemical
experiments performed by Keiko Yokoyama.
Not satisfied with merely identifying this new copper site, Gray and company
sought the assistance of Serena DeBeer George, then affiliated with SSRL and
now an assistant professor at Cornell University, to
gain a more comprehensive understanding of the electronic structure of the
"type zero" mutants. Copper K-edge XAS measurements at Beam Line 7-3 yielded
two insights. First, EXAFS data demonstrated that the crystallographically
determined structures indeed corresponded to the copper(II) sites, thus
absolving any fears of photoreduction during data collection [Figure 1]. The
XANES data corroborated this observation, showing an intensity increase in the
1s to 3d ligand-field transition that tracked with the crystallographically
determined distortion toward tetrahedral site geometry [Figure 2]. It also
indicated that the narrow A|| observed in the "type zero" EPR spectrum is not
attributable to this tetrahedral distortion, a hypothesis initially proposed
for T1 copper site EPR behavior.
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.