Researchers Visualize the Structural Intermediates of the Nickel-catalyzed
Enzyme that Makes Methane
summary written by Raven Hanna
In the atmosphere, methane is a much more potent greenhouse gas than carbon dioxide, trapping 20 times more heat. As a fuel, methane burns cleanly, producing less carbon dioxide per unit of heat than other fuels. For these reasons, understanding methane production is immediately important.
All of the biologically produced methane comes from methanogenic archaea bacteria. These methanogens produce methane using an enzyme called methyl-coenzyme M reductase. MCR's complex reaction requires a reduced nickel cofactor in the enzyme's active site. Researchers have proposed two competing theories of the enzyme's mechanism that differ in the chemistry of the first step of the reaction.
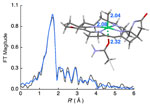
A group of researchers led by SSRL's Ritimukta Sarangi used Beam Line 9-3 to acquire structural information for the MCR's reaction intermediates to determine which mechanistic theory is correct. Overcoming difficulties due to MCR's sensitivity to oxygen, the researchers used a combination of Ni K-edge x-ray absorption spectroscopic (XAS) studies and density functional theory (DFT) calculations to find the electronic and geometric properties of various enzyme-intermediate complexes.
Through these experiments, the researchers identified which of the proposed mechanistic theories is correct. They also found that certain traits of the nickel cofactor help stabilize the potentially unstable reaction intermediates. This work was published in the April issue of the journal Biochemistry.
To learn more about this research see the full Scientific Highlight
Sarangi, R.; Dey, M.; Ragsdale, S. W. Geometric and electronic structures of the Ni(I) and methyl-Ni(III) intermediates of methyl-coenzyme M reductase. Biochemistry 2009, 48, 3146-3156.