
Tomohisa Kuzuyama1,2, Joseph P. Noel1 & Stéphane B. Richard1
1Jack Skirball Chemical Biology and Proteomics Laboratory, The Salk
Institute for Biological Studies, 10010 North Torrey Pines Road, La Jolla,
California 92037, USA
2Laboratory of Cell Biotechnology, Biotechnology Research
Center, The University of Tokyo,
1-1-1 Yayoi, Bunkyo-ku, Tokyo 113-8657, Japan
Prenylation is a general term for the chemical or enzymatic addition of a
hydrophobic isoprenoid side chain to an accepting molecule (another isoprenoid
chemical, small aromatic molecule, protein, etc). Prenylation of aromatic
natural products plays a critical role in the biosynthesis of chemically
complex and structurally diverse molecules playing important biological
functions across a wide phylogenetically diverse group of organisms, from
bacteria to mammals. Hybrid natural products such as the anti-oxidant
naphterpin1-3 that contain a
polyketide core decorated with 5-carbon (dimethylallyl), 10-carbon (geranyl) or
15-carbon (farnesyl) isoprenoid chains possess biological activities distinct
from their non-prenylated aromatic precursors4.
These hybrid natural products represent new anti-microbial, anti-oxidant,
anti-inflammatory, anti-viral and anti-cancer compounds.
Enzymes capable of regiospecific prenylation of bioactive compounds will serve
as novel chemoenzymatic tools for natural product diversification and the
chemo-enzymatic development of therapeutically novel synthetic compounds.
We recently reported the gene identification, biochemical characterization and
high resolution crystal structure of an architecturally novel aromatic
prenyltransferase (PTase), Orf2 from Streptomyces sp. strain CL190. This
protein belongs to a recently identified and completely new class of aromatic
prenyltransferases showing no sequence similarity with any protein
prenyltransferase5. The three dimensional structure
of Orf2 consists of a single domain possessing a novel barrel fold (Fig. 1).
This new barrel, termed a PT-barrel for
Prenyltransferase-barrel, is a
cylindrical b-sheet comprising ten anti-parallel
b-strands arranged around a central solvent filled
core. In an arrangement reminiscent of a TIM-barrel, the cylindrical b-sheet is surrounded by a ring of solvent exposed
a-helices; however, the connectivity and
directionality of Orf2's secondary structure elements is not shared with the
TIM-barrels, b-barrels or even the
dimeric a/b-sandwich structural families (Fig. 1).
Using X-ray diffraction data collected at SSRL (BL9.2), ESRF (FIP/BM30A) and
BNL (X8C,X6A), we determined four X-ray crystal structures of Orf2
substrate/substrate analog complexes, including Orf2 bound to a TAPS buffer
molecule, a binary Orf2 complex containing geranyl diphosphate (GPP) and
Mg2+,
a ternary Orf2 complex containing a non-hydrolyzable GPP analog, geranyl
S-thiolodiphosphate (GSPP), Mg2+ and 1,6-DHN, and a ternary Orf2
complex containing GSPP, Mg2+ and flaviolin (Fig. 2).
Opposite to most previously identified membrane bound aromatic
prenyltransferases, this bacterial enzyme is a small soluble monomeric protein.
In vivo, Orf2 attaches a geranyl group to a
1,3,6,8-tetrahydroxynaphthalene
(THN) derived polyketide during naphterpin biosynthesis. In vitro, Orf2
catalyzes carbon-carbon and carbon-oxygen based prenylation of a diverse
collection of hydroxyl-containing aromatic acceptors of synthetic, microbial
and plant origin. This in vitro activity against plant derived natural products
is the first demonstration of an enzyme activity capable of forming a
carbon-oxygen prenyl linkage.
The resultant PTase activity of Orf2 displays promiscuous but regiospecific
prenylation activity against diverse aromatic substrates: stilbenes,
flavonoids, isoflavonoids and related plant polyketides including naringenin,
resveratrol as well as olivetol and olivetolic acid. These later two plant
polyketides and their geranylated products serve as intermediates in the
biosynthesis of the plant derived polyketide-terpene natural product
D9-tetrahydrocannabinol
(D9-THC)6. For the isoprenoid diphosphate
substrates, Orf2 exhibits no activity with dimethylallyl diphosphate (DMAPP),
the highest relative Mg2+ dependent activity with geranyl
diphosphate and detectable activity with farnesyl diphosphate (FPP).
These crystal structures coupled with in vitro assays and the modeling of other
known aromatic prenyltransferases provide a basis for understanding and
manipulating the regio-specific prenylation of aromatic small molecules using
this structurally unique family of aromatic PTases.
Primary Citation
References


Figure 1. Topology diagram and overall structure of the Orf2 aromatic PT-barrel
fold complexed with a non-hydrolysable geranyldiphosphate analog (GSPP),
Mg2+ and 1,6-dihydroxynaphtalene molecule (DHN2).
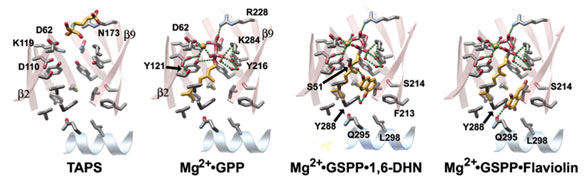
Figure 2. Orf2 substrate/substrate analog complexes. Only the b-strands (minus -strands 1 and 10) plus the C-terminal a-helix are displayed in the same vertical orientation as
in a after a 30o counter-clockwise rotation
around the barrel axis. Hydrogen and coordination bonds are shown as green
spheres.
Kuzuyama T, Noel JP & Richard SB (2005) Structural basis for the promiscuous
biosynthetic prenylation of aromatic natural products.
Nature
435, 983-7.
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |