

 | |
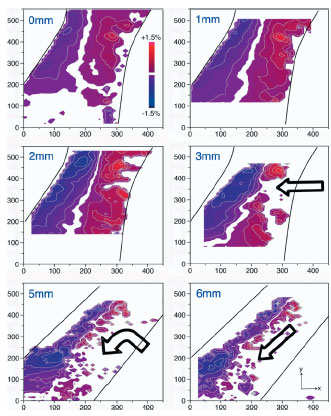
| Fig. 1: Maps of the deviatoric strain of the B2 austenite along the vertical y axis (eyy) from x-ray diffraction analysis. The global displacements for these maps are shown in the upper left corner of each image. The dark lines illustrate the approximate location of the edges of the diamond strut. The red color indicates tensile strain whereas blue indicates compressive strain; note also the presence of a white neutral axis. The maximum local strain in austenite was measured to be ±1.5%. Above this strain, austenite transforms to martensite, which for this analysis is not analyzed, but can be inferred from where the austenite disappears. Martensite generally initiates at the surfaces with the highest applied deformation and begins to move toward the center of the diamond strut, as indicated by the arrows in 3mm, 5mm, and 6mm maps. However, it is observed that, even at 6mm deformation, there is a region of strain-stabilized retained austenite along the center of the strut that resists transformation. Consequently, the martensite transformation front moves down along the strut edge as deformation strains increases. | |
Nitinol, a nearly equiatomic alloy of nickel-titanium, can "remember" a
previous shape and can recover strains as high as 10% by deformation
(superelasticity) or temperature change (shape memory). These properties
result from a reversible first-order phase transition between austenite (cubic,
B2) and martensite (monoclinic, B19'). As such, deformation mechanisms of
Nitinol are more complex than the conventional modes of plastic deformation in
traditional alloys. Consequently, the mechanical behavior of Nitinol under
multiaxial conditions remains poorly understood.
Nevertheless, because of these unique mechanical characteristics, in
combination with excellent biocompatibility, Nitinol is used as self-expanding
endovascular stents to scaffold diseased peripheral arteries. First-generation
Nitinol stents were designed to provide sufficient scaffolding forces to hold
open vessels, yet provide enough elasticity to "breathe" with pulsatile
pressure differentials from the cardiac cycle. A variety of clinical studies
indicate that these stents perform this primary function quite well. More
recent in-depth studies, however, reveal that superficial femoral arteries
(SFAs) are subjected to complex in vivo multiaxial deformation with up to 60%
rotation and ~20% contraction in the SFA as the leg is bent from an extended
position. Correspondingly, during a walking cycle, a stent deployed in the SFA
undergoes severe multiaxial displacements from pulsatile motion
(~4x107 cycles
annually) plus bending, torsion, and axial motions (at a rate of
~1x106 cycles
annually). Although there are ~40 times more cardiac displacement cycles, the
combined non-pulsatile motions result in far greater cyclic strain magnitudes,
and therefore, have the possibility of inducing greater fatigue damage, and
sometimes fracture.
We investigated deformation of a stent-like component under moderate to high
deformation conditions in an in situ apparatus at ALS BL 7.3.3, using a 1x1
micron white x-ray beam. The sample (the whole loading apparatus) was rastered
to cover a 400x600 microns region on a 7x7 micron grid. A white beam Laue
diffraction pattern was collected at each point on the grid. We were able to
index the Laue pattern to obtain the all the 6 elements of the 2nd rank
deviatoric strain tensor. Local strain maps thus obtained at several
deformation levels were compared with a strain map obtained from the
state-of-art finite element (FE) model used for Nitinol stent design.
Besides local granularities the strain maps from microdiffraction agreed well
with FE model at low deformations, but difference between them increased
dramatically at high deformation values. For example, contrary to the model
predictions, there remains a spine of retained austenite in the middle of the
strut even at the highest deformation studied here. Accordingly, it is observed
that the transformation front moves down the length of the strut rather
than moving towards the middle (3 - 5 mm).
The experimental observation of redirection of the transformation front has
very important implications for strut fractures in Nitinol stents. At
relatively low displacements, if the local strain field encounters a
sufficiently large flaw, the defect will induce the strain field to grow such
that the material may fracture at the location predicted by the FE model.
Therefore, in this regime fracture of Nitinol stents is very well predicted by
the current models. However, in the absence of flaws near the initial
high-strain region predicted by FE model, the transformation front, which has
now changed direction, will begin to propagate down the length of the strut.
As the propagating strain front runs down the length of the strut, fracture may
occur at locations significantly different from those suggested by the
finite-element model, making fracture of SFA stents under non-pulsatile
overload conditions unpredictable.
These results show that a much better understanding of crack propagation and
modes by which superelastic Nitinol accommodated high deformation is needed.
We are in process of publishing a series of investigations which sheds some
light on these fundamental deformation and fracture processes in superelastic
materials.
Primary Citation
A. Mehta, X.-Y. Gong, V. Imbeni, A. R. Pelton and R. O. Ritchie, "Understanding
the Deformation and Fracture of Nitinol Endovascular Stents Using In
Situ Synchrotron X-ray Microdiffraction", Adv. Mater. 19,
1183 (2007)
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |