

Complex Fe-S cluster-containing enzymes are ubiquitous in nature where they are
involved in a number of fundamental reactions for life including carbon dioxide
fixation, nitrogen fixation, and hydrogen metabolism. One of the more complex
and unusual biological clusters is found in the [FeFe]-hydrogenase. The
active-site H-cluster in these enzymes has a [4Fe-4S] subcluster bridged via a
cysteine thiolate to a 2Fe subcluster, which in turn is coordinated by CO and
CN- ligands and a bridging dithiolate ligand (1).
The biologically unique CO and CN- ligands finely tune the 2Fe
subcluster of the H-cluster making it able to efficiently catalyze the
activation of molecular H2 through the reversible reaction
H2
Figure 1. Top:
overall ribbon and surface representation of the x-ray crystal structure of
[FeFe]-hydrogenase HydAdeltaEFG. Loop regions important for the
cluster insertion process are colored green. Bottom: Ball and stick
representation of the active site of HydAdeltaEFG at which a [4Fe-4S] cluster is
present. Coloring scheme: rust, iron; orange, sulfur; gray, carbon; red,
oxygen; blue, nitrogen.
The crystal structure of HydAdeltaEFG reveals that the [4Fe-4S]
cluster is the only Fe-S cluster present at the active site and the 2Fe
subcluster of the H-cluster is absent (Figure 1). The overall structure of
HydAdeltaEFG reveals the formation of a positively charged channel
from the protein surface linked to the [4Fe-4S] cluster site (Figure 2).
Adjacent to the [4Fe-4S] cluster is an open cavity, which is poised for
insertion of the 2Fe subcluster. Presumably, insertion of the 2Fe subcluster
occurs through the positively charged channel that collapses following
incorporation through conformational changes two loop regions (Figure 2).
These observations suggest a stepwise mechanism for H-cluster biosynthesis and
that the [4Fe-4S] subcluster is synthesized and inserted first by general Fe-S
cluster maturation machinery followed by the synthesis and insertion of the 2Fe
subcluster by specialized hydrogenase maturation machinery. Interestingly, a
sequence alignment of a diversity of HydA indicates that the two loop regions
implicated to be involved in the cluster insertion process are highly conserved
and a phylogenetic analysis suggests that HydA emerged within bacteria most
likely from a Nar1p-like ancestor lacking the 2Fe subcluster, followed by
acquisition in several lower order eukaryotes.
Figure 2. Pathway for Fe-S cluster insertion into
[FeFe]-hydrogenase during complex Fe-S cluster assembly. The surface
representation of HydAdeltaEFG is gray (top) and loop regions
important for cluster insertion are overlayed from the active
[FeFe]-hydrogenase (HydA). Below are electrostatic surface representations of
HydAdeltaEFG (left) and HydA (right).
This work was supported by an AFOSR Multidisciplinary University Research
Initiative Award (FA9550-05-01-0365) and the NASA Astrobiology Institute
(NAI)-Funded Astrobiology Biogeocatalysis Research Center (NNA08C-N85A).
Primary Citation
Mulder, D. W., Boyd, E. S., Sarma, R., Lange, R. K., Endrizzi, J. A.,
Broderick, J. B., and Peters, J. W. (2010) Stepwise [FeFe]-hydrogenase
H-cluster assembly revealed in the structure of HydAdeltaEFG,
Nature doi:10.1038/nature08993.
References
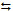 2H+ + 2e-. How this complex
metallocluster is assembled in nature is intriguing and the precise mechanism
by which various enzymes, scaffolds, and carriers carry out H-cluster
maturation is unknown.
2H+ + 2e-. How this complex
metallocluster is assembled in nature is intriguing and the precise mechanism
by which various enzymes, scaffolds, and carriers carry out H-cluster
maturation is unknown.
We have determined the x-ray crystal structure of an intermediate form of
[FeFe]-hydrogenase (HydAdeltaEFG) from the green algae
Chlamydomonas reinhardtii expressed in Escherichia coli in a
genetic background devoid of the hydrogenase genes required for maturation.
The x-ray crystal structure of HydAdeltaEFG was refined to 1.97
Å resolution from data collected at Beam Lines 9-1 and 9-2 at SSRL.
Insights from the structure establish unifying themes for the assembly of
complex Fe-S clusters in nature and reveal fundamental features of
metalloenzyme evolution by providing clues as to how these clusters have
evolved in complexity through time. Also, the research, by revealing key
insights into the biosynthesis of a biological catalyst (the H-cluster) that
promotes hydrogen production, provides clues into approaches for biomimetic
catalysts for hydrogen as a renewable energy source.

The structure of HydAdeltaEFG also establishes a parallel to the
cluster assembly process for nitrogenase and indicates that the cluster
insertion mechanism is conserved between these two evolutionary distinct
enzymes. For nitrogenase, structural determination of a FeMo-co deficient form
of the enzyme reveals major structural rearrangement in the domain involved in
FeMo-co binding, resulting in a positively charged channel linked to the
FeMo-co site (2). The structural rearrangements resulting in the formation of
a positively charged channel in both [FeFe]-hydrogenase and nitrogenase
indicate that the process for complex Fe-S cluster insertion into proteins may
be conserved and that the evolution of functional [FeFe]-hydrogenase may have
occurred stepwise, a feature which is consistent with the evolution of
nitrogenase and possibly Fe-S enzymes in general. Also, like
[FeFe]-hydrogenase, different Fe-S cluster components of nitrogenase are
targets of different maturation machinery-general and specialized nitrogenase
maturation machinery. These parallels suggest that there are unifying themes
by which biology constructs complex Fe-S clusters and gives us insight into
which components are likely to be the youngest and which might be the oldest in
the quest to trace their evolutionary origin.

SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.