The Structure of an Algal Hydrogenase Reveals the Assembly and Evolution of
Complex Metalloenzymes
summary written by Raven Hanna
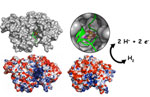
The potential for using biological enzymes to make hydrogen to use as a renewable energy source is a hot topic, but little is known about how these complex enzymes assemble and work. The [FeFe]-hydrogenase enzyme binds iron and sulfur ions to catalyze the reversible production of hydrogen ions from protons and electrons. The enzyme's active site, termed the H-cluster, uses a complex Fe-S cluster comprised of a [4Fe-4S] subcluster and a 2Fe subcluster to catalyze the reaction.
A group led by John Peters of Montana State University used SSRL Beam Lines 9-1 and 9-2 to solve the crystal structure of a green algae [FeFe]-hydrogenase enzyme. They used an intermediate form of the enzyme to acquire information about the mechanism of enzyme assembly and maturation. Their 1.97 Å structure revealed a positively charged channel linked to the [4Fe-4S] subcluster site, while the 2Fe subcluster was absent. The authors propose a stepwise mechanism in which the [4Fe-4S] subcluster is assembled first and then the 2Fe cluster is assembled and inserted through the channel, which collapses and disappears in the mature enzyme. This mechanism is similar to that seen in nitrogenase, which transforms nitrogen gas into ammonia, suggesting an evolutionary link.
The researchers suggest that knowledge obtained from this structure can inform efforts to engineer better hydrogenases, ultimately leading to more cost-effective renewable energy sources, such as hydrogen fuel cells. This work was published on April 25 on the Nature website.
To learn more about this research see the full Scientific Highlight
Mulder, D. W., Boyd, E. S., Sarma, R., Lange, R. K., Endrizzi, J. A., Broderick, J. B., and Peters, J. W. (2010) Stepwise [FeFe]-hydrogenase H-cluster assembly revealed in the structure of HydAdeltaEFG, Nature doi:10.1038/nature08993.