

Carbon-based materials have long been used for a variety of water purification
operations. Researchers have investigated carbon materials as adsorbents for
decades, but only limited information on the precise details of aqueous ion
interactions with carbon surfaces has been uncovered. It is empirically known
that the affinity of activated carbon for various hydrated ions depends
critically on how the material is processed. Processing influences the types of
chemical groups and the structure of the carbon surface, which in turn
influences the strength of interaction between hydrated ions and the carbon
surface. It is also believed that many of the puzzling properties of
impurity-free carbon, such as ferromagnetism, are governed by specific
modifications of the carbon surface. However, very little is known about the
local structure of the carbon surface that is responsible for its aqueous ion
affinity.
In a recent paper published in Advanced Materials, former Lawrence Livermore
National Laboratory scientist Jason Holt, along with SSRL scientists Apurva
Mehta, Erik Nelson, and Samuel Webb have found that both activated carbon and
carbon nanotubes share a common surface site that binds bromide ions present in
solution, and by implication other aqueous halides as well. These sites have a
significant impact on the chemical properties of these materials and provide a
picture of carbon surface chemistry that is consistent with the proposal of a
recent theoretical study.[1]
To probe the details of ion structure, a technique was needed that is sensitive
to the local environment around ions that may be trace in abundance in samples.
The technique of extended x-ray absorption fine structure (EXAFS) is ideally
suited for this purpose, and beamlines 10-2 and 7-3 at the Stanford Synchrotron
Radiation Laboratory (SSRL) were used to carry out these measurements. Carbon
materials were prepared for these measurements by taking them in powdered form
and treating them with a concentration solution of rubidium bromide, followed
by a series of washing cycles to remove excess ions present in the bulk
solution.
Figure 1 shows the bromine EXAFS oscillations, k3C(k), for both a bulk
solution
sample (0.5M rubidium bromide) and an activated carbon sample (AC6), their
pseudoradial distribution functions (R-function), as well the best model fits
for k3C(k) and the
R-functions. The k-space EXAFS of the bulk solution dies out
rapidly by k~10Å-1, indicating significant disorder in the outer coordination
shells. This suggests that the interaction of the Br with more distant water
molecules is very weak (and probably strongly screened by the first
coordination shell). In contrast, the EXAFS of the activated carbon sample
shows a beating of several frequencies and extends out beyond
k=13Å-1,
indicating that Br binds to a very ordered structure in a very specific manner.
The resulting R function is also much more complex, with multiple peaks
observed, suggesting that the local structure around a Br ion in the activated
carbon sample is dramatically different from that in aqueous solution. Similar
results were seen with single wall carbon nanotube samples that were analyzed.
Because several close and partially overlapping scattering shells were visible
in the experimental EXAFS data, due to Br attachment to the graphitic material,
fitting consisted of creating a rigid graphitic sheet and refining the position
of Br over this sheet. There are two general types of locations available for
an adsorbate on a graphitic sheet. The first places the Br ion in the middle of
a complete graphitic sheet, either towards the broad end of the carbon ring or
nearer to the triangular portion of the carbon ring. The second general
location for an adsorbate is at the edge of the sheet. The first type of edge
site corresponds either to the concave or the convex portion of the broad part
of a "broken" carbon ring that has a 4 carbon periodicity (the "armchair"
site). The second edge location corresponds to the concave or convex corner of
the triangular part of the "broken" carbon ring. Such an edge has a 2 carbon
periodicity and is referred to as a "zigzag site". After considering all
candidate locations and examining their fit statistics, it was concluded that
the bromine ion does not attach to the middle of the graphitic sheet but to an
edge site, with a strong preference for a convex, zigzag edge, as illustrated
schematically in Figure 2. Note that in addition to actual edges of graphitic
sheets, these edge sites can be in the middle of a graphitic sheet, or on the
side of a carbon nanotube, provided the sheet or tube is punctured (i.e.,
vacancies are present). A recent theoretical study [1] proposes an oxygen-free
basic site, the carbene site at graphene zigzag edges that appears consistent
with the experimental results reported here. These sites can be protonated in
aqueous solution, producing a positively charged carbon with which anions may
interact through ion pairing.
In conclusion, the present results provide the first structural picture of the
location and binding geometry of aqueous halogen ions to a graphitic sheet.
The results also suggest how the presence of zigzag graphitic edge sites can
alter the ion affinity. These particular sites may prove important for
proposed uses of carbon nanotube membranes [2] for desalination, as well as
carbide-derived carbons for electrolytic supercapacitors.[3]
Primary Citation:
References:
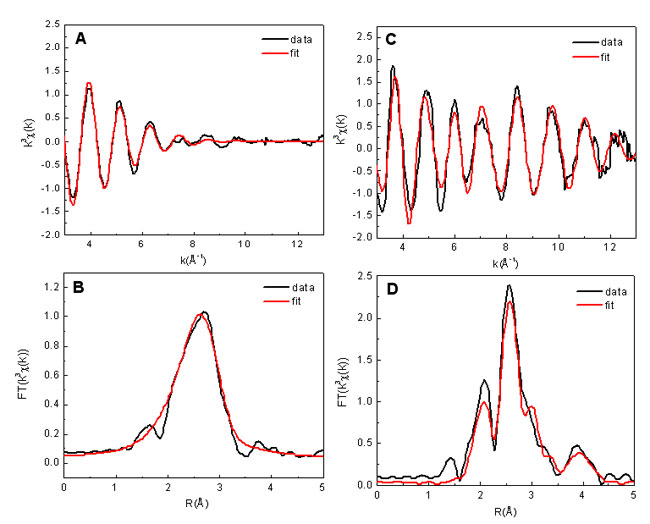
Figure 1.
Bromine EXAFS signal (A) and the R-function (B) for the 0.5M rubidium bromide
reference solution. Corresponding bromine EXAFS (C) and R-function (D) for the
activated carbon sample, along with model fits (in red) derived from FEFF8.[3]
The fit was performed over a k-space from 3 to 12.7 Å-1
(R from 1 to 4.7 Å) for
the solution sample and the AC6 sample. The difference between the peak
positions and the true Br coordination distances can be resolved by a phase
correction term.
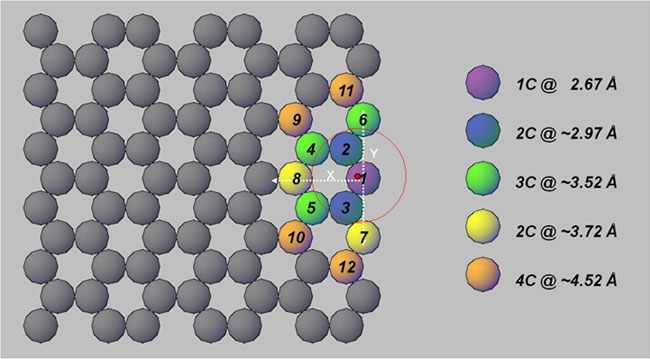
Figure 2.
Illustration of the structural model for Br coordination to graphene materials,
derived from a model fit of the activated carbon EXAFS data. Bromine sits atop
a convex zigzag site on the edge of the graphene sheet (red dot above carbon
atom #1). The Br position relative to the axes is x = 0.199±0.040Å, y =
0.076±0.029, z = 2.658±0.008, where z is the direction above the graphitic
sheet.
A. Mehta, E.J. Nelson, S.M. Webb, and J.K. Holt, Adv. Mat.,
published online 10/30/08; DOI: 10.1002/adma.20080160
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.