Ions in the Clutches of Carbon Nanotubes
Currently, more than 1/6th of the people in the world lack safe and clean water. Population shifts, global warming and many other global changes will make this shortage even more critical in the coming years. Solutions to this problem lie in the development of new, energy-efficient means of purifying water. One of the traditional purification technologies—called membrane-based reverse osmosis—is very energy intensive, and this is spurring a flurry of activities into creating new, energy-efficient materials. Among the most promising of these materials are carbon nanotubes. A related material, activated carbon—basically carbon treated at extreme temperatures to achieve a high degree of microporosity and functionality-has been used for a century or more to filter water, and soak up unwanted compounds. However, the precise arrangements of the atoms that give rise to these purifying properties have mostly remained a mystery.
The most detailed picture yet of how specially arranged carbon atoms interact with and adsorb ions has emerged from new research conducted in part at SSRL Beam Lines 10-2 and 7-3. Using Extended X-ray Absorption Fine Structure (EXAFS) researchers looked at the arrangement of bromine ions near the surfaces of carbon nanotubes and activated carbon. The results were published in the October 30, 2008 online edition of Advanced Materials.
Carbon nanotubes are created when extremely thin sheets of graphite are rolled
into tubes with walls one atom thick, and a diameter as small as a nanometer.
Although they do possess many subtle and important differences, nanotubes
appear to share many of activated carbon's unique properties in the presence of
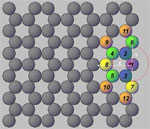
ions. The recent study sheds light on what researchers term "zigzag" sites
present on the nanotube's surface, made up of carbon atoms configured in such a
way as to attract and hold on to ions. These results help researchers better
understand the surface chemistry of carbon nanotubes and potentially tailor it
for a range of applications, from electronics to environmental science.
To learn more about this research see the full Scientific Highlight
A. Mehta, E. J. Nelson, S. M. Webb, and J. K. Holt, Adv. Mat., published online 10/30/08; DOI: 10.1002/adma.20080160