
Starting in the 1970s, federal regulatory control and eventual elimination of
lead-based "anti-knock" additives in gasoline decreased the level of airborne
Pb in the USA by two orders-of-magnitude [1]. Blood lead levels
of the USA
|
|
 |
|
Figure 1. The good, the bad, and the ugly.
Ambient airborne particulate matter captured on filters of woven silica fiber
(large strips) and TeflonTM (round). Clean fiber filter at bottom
for comparison. Take a deep breath?
|
|
population decreased correspondingly [2,3].
Despite this dramatic improvement in both exposure risk and body burden of Pb,
the sources and health threat of the low levels of lead in our "unleaded" air
remain topics of scientific and public health interest [4,
5]. Lead is a potent
neurotoxin in children, particularly for toddlers whose brains are developing
and who often are exposed to lead through hand-to-mouth transfer. Some
researchers posit that there is no safe minimum exposure.
Societal decisions on regulation of airborne lead involve not just medical
knowledge, but also an understanding of the sources of airborne lead, the
cycling of lead in urban settings, and human exposure routes. Mobile
(vehicular) sources represented the dominant Pb input through much of the
20th Century. With the elimination of leaded gasoline, focus in
developing a new air standard for Pb has been on such point sources as
smelters, lead recycling operations, manufacturing, combustion, and the
continued use of leaded fuel for aviation piston engines [6].
Late last year the US Environmental Protection Agency (EPA) unveiled their new
airborne particulate standard for lead, 0.15 mg Pb
m-3 air, averaged over rolling 3-calendar-month periods [6-8]. This is an order-of-magnitude decrease
from their 1970s-era standard of 1.5 mg Pb
m-3 air.
In spite of this progress, it was reported that other researchers and even some
members of the EPA's scientific advisory panel urged stricter limits, as low as
0.02 mg Pb m-3 air
[8,9].
Instead of considering potential sources of airborne lead, a multi-disciplinary
team of environmental and health scientists from the University of Texas at El
Paso posed the question: what species (compounds) of Pb are actually
present in our air? Their approach, using x-ray absorption spectroscopy, was to
identify and, if possible, quantify the major species Pb in ambient airborne
particulate matter collected in El Paso, TX, USA. The diverse team included a
geochemist, a nurse, an engineer, an environmental sciences graduate student,
and a retired air-quality manager.
The team examined 20 samples of particulate matter (PM) collected on woven
silica fiber filters in 2005 and 1999 at three sites in El Paso. The XAS
experiments, conducted on Beam Lines 7-3, 10-2, and 11-2 at SSRL, proved
challenging: the amount of Pb that was exposed to the beam was in the
nanogram-to-microgram range. Nonetheless, it became clear that the PM samples
exhibited the same overall XAFS (x-ray absorption fine structure) structure.
That "fingerprint" also proved similar to those of soil samples collected
nearby (Fig. 2). Comparison of the PM spectral fingerprint to those of common
forms of environmental Pb turned up one prime suspect, Pb-humate (Fig. 3). Lead
humate is a stable complex of Pb sorbed to humic acid; it forms exclusively in
the humus fraction of soils. In Pb-humate, near-neighbor shells to the sorbed
Pb are populated by O and C atoms; the lack of strong backscatterers (e.g., Pb,
as in crystalline, space-repetitive Pb salts) in farther shells yields a simple
spectrum and Fourier transform.
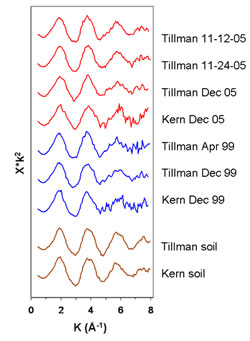 | |
Figure 2. Pb-LIII XAFS spectra of PM samples and associated
local soils. Spectra are background-subtracted, normalized,
k2-weighted, and plotted in k-space. Because the
small amounts of Pb in the PM produced noisy XAFS, multiple abbreviated scans
were stacked. Since we sampled only a short region of k-space, the
XANES and EXAFS regions are presented together as a single XAFS fingerprint.
Tillman and Kern are old neighborhoods in the urban core of El Paso with
long-term PM collection stations. (Figure adapted from Pingitore et al. 2009)
|
|
|  |
|
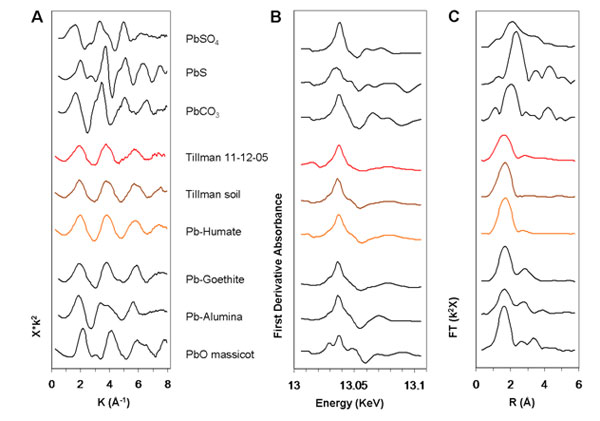
Figure 3: Pb-LIII XAFS and derived spectra of model compounds,
PM, and
soil. (A) XAFS spectra. Spectra are background-subtracted, normalized,
k2-weighted, and plotted in k-space.
(B) Derivatives of Pb-LIII XANES spectra
of several samples and model compounds. Derivatives were taken of spectra that
had been background-subtracted, normalized, and k2-weighted.
(C) Radial
distribution functions (RDF) derived from Fourier transforms of
Pb-LIII XAFS
spectra of samples and model compounds. No distance correction for the phase
shift during photo-electron backscattering was applied. (Figure adapted from
Pingitore et al. 2009) |
|
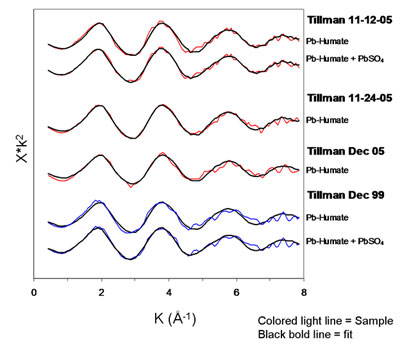
Least-squares fits of Pb-humate to the El Paso PM are striking (Fig. 4). In
some samples a small amount of lead sulfate may also be present. Lead humate
also provided near perfect fits to the local soil samples. Despite these
compelling fits, it is possible that sorption complexes on other
low-atomic-number materials (e.g., some clays) could provide computationally
equivalent matches and be present in the PM. This would not, of course, have
bearing on the match observed between PM and soil.
|  |
|
|
Figure 4. Least-squares fits of XAFS spectra of PM with Pb-humate and
Pb-humate + PbSO4. The contribution of PbSO4 to the
fit in 2 cases was ~ 15%. In the other two cases the lead sulfate did not
improve the fit noticeably and was omitted. (Figure adapted from Pingitore et
al. 2009) |
|
The study concluded that local Pb-contaminated soils, father than current
industrial emissions, are the dominant source of Pb in contemporary airborne
particulate matter in El Paso, TX. This provides direct evidence for an earlier
suggestion that soil lead might explain the large discrepancy in mass-balance
input-outflow models of Pb in the Los Angeles air basin [7].
Thus, earlier anthropogenic Pb releases left a legacy of contaminated soils
that now leak Pb into our air in a perverse form of recycling. The process will
continue to do so for the foreseeable future.
From a health standpoint, the typical US inhabitant actually takes in very
little Pb by simply breathing, compared to such other activities as eating. The
real risk of airborne Pb is for toddlers, crawling on floors contaminated with
lead-bearing dust, the indoor fallout from resuspended Pb-contaminated soil.
The bottom line? At some point, meeting further legislative restriction of Pb
in airborne particulate matter could require the remediation or removal of
Pb-contaminated soil to prevent its resuspension into the air. That will prove
very expensive. And for those toddlers, worth it.
Primary Citation
Pingitore Jr NE, Clague J, Amaya MA, Maciejewska B, Reynoso JJ (2009) Urban
Airborne Lead: X-ray Absorption Spectroscopy Establishes Soil as Dominant
Source. PLoS One 4(4): e5019. doi:10.1371/journal.pone.0005019
http://www.plosone.org/article/info:doi/10.1371/journal.pone.0005019.
References
[1] Davidson CI, Rabinowitz M (1992) Lead in the environment: From sources
to human receptors. In: Needleman HL, editor. Human Lead Exposure. Boca Raton:
CRC, pp. 65-86.
[2] Annest J, Dirkle J, Makuc C, Nesse J, Bayse D, et al. (1983)
Chronological trend in blood lead levels between 1976 and 1980. N Engl J Med
308: 1373-1377.
[3] ATSDR (Agency for Toxic Substances Disease Registry) (1988) The Nature
and Extent of Lead Poisoning in Children in the United States: A Report to
Congress. Atlanta: US Dept of Health and Human Services, Public Health Service,
ATSDR.
[4] Anon (2007) Tougher standard for lead in air recommended. Chem Eng News
85(46): 39.
[5] Stokstad E (2008) Panel: EPA proposal for air pollution short on
science. Science 319: 146.
[6] US Environmental Protection Agency (2008c) Environmental Protection
Agency 40 CFR Parts 50, 51, 53 and 58 [EPA-HQ-OAR-2006-0735; FRL-_____- _] RIN
2060-AN83 National Ambient Air Quality Standards for Lead. Available:
http://epa.gov/air/lead/pdfs/20081015_pb_naaqs_final.pdf [accessed 7 November
2008].
[7] Harris AR, Davidson CI (2005) The role of resuspended soil in lead
flows in the California South Coast Air Basin. Environ Sci Technol 39:
7410-7415.
|





