

Junko Yano1,2, Jan Kern3, Klaus-Dieter Irrgang3, Matthew J. Latimer4, Uwe Bergmann4, Pieter Glatzel5, Yulia Pushkar1,2, Jacek Biesiadka6, Bernhard Loll6, Kenneth Sauer1,2, Johannes Messinger7, Athina Zouni3, Vittal K. Yachandra1
1Melvin Calvin Laboratory, Physical Biosciences Division, Lawrence
Berkeley National Laboratory, and 2Department of Chemistry,
University of California, Berkeley, CA, USA
3Max-Volmer-Laboratorium für
Biophysikalische Chemie, Technische Universität, and 6 Institut für
Kristallographie, Freie Universität, Berlin, Germany
4Stanford Synchrotron
Radiation Laboratory, Menlo Park, CA, USA
5European Synchrotron Radiation
Facility, Grenoble Cedex, France
7Max-Planck-Institut für Bioanorganische
Chemie, Mülheim, Germany.
All complex life forms on earth rely on oxygen. Only because of constant
regeneration of oxygen through photosynthetic water oxidation by green plants
and cyanobacteria is this all important substance abundant in the atmosphere.
Yet, it is still not known how exactly this reaction is carried out. The
structure of the catalytic Mn4Ca complex that is part of a
multi-protein membrane system known as photosystem II (PS II), that performs
the reaction of splitting and oxidation of water has been the subject of
intense study ever since Mn was identified as an essential element. The
Fig. 1. Mn XANES and EXAFS of single crystals of photosystem II as a
function of x-ray dose. As the x-ray dose increases, Mn in PS II normally
present as Mn4(III2,IV2) is reduced to
Mn(II) as seen by the changes in XANES spectra (left bottom). The changes in
the corresponding EXAFS spectra (right) show that the three Fourier peaks
characteristic of Mn-bridging-oxo, Mn-terminal, and Mn-Mn/Ca interactions
(dashed vertical line) are replaced by one Fourier peak characteristic of a
Mn(II) environment. A PS II crystal subsequent to x-ray exposure is also shown
(left top). The bright green color is from the chlorophyll molecules in PS II
and the dark spots show the areas of the crystal used for x-ray diffraction
data collection.
In this study, x-ray absorption spectroscopy was used at the Mn K-edge to
systematically investigate x-ray induced radiation damage to the oxygen
evolving Mn4Ca site of PS II single crystals. The study, led by a
group from LBNL's Melvin Calvin Laboratory, involves a collaborative effort
between groups involved in x-ray spectroscopy (LBNL, Max-Planck-Institut für
Bioanorganische Chemie, Mülheim, ESRF and SSRL) and x-ray crystallography of PS
II (Technische Universität and Freie Universität, Berlin). The methodology for
collecting single crystal XAS data from PS II was developed in collaboration
with the Structural Molecular Biology group at SSRL.
The Mn XANES data from PS II single crystals show that following x-ray doses
characteristic of the recently published x-ray diffraction measurements, the Mn
is largely reduced to Mn(II) from
Mn4(III2,IV2) present in the native
dark-adapted PS II complex (S1-state). Moreover, the EXAFS spectrum changes
significantly, from one that is characteristic of a high-valent multinuclear
oxo-bridged Mn4Ca complex to one that is typical of mononuclear
hexa-coordinated Mn(II) in solution (Fig. 1). These studies reveal that the
conditions used for structure determination by x-ray crystallography cause
serious damage specifically to the metal-site structure, and provide
quantitative details. The results show furthermore that the damage to the
active metal site occurs at a much lower x-ray dose compared to the loss of
diffractive power of the crystals as
established by x-ray crystallography. The damage is significantly higher at
wavelengths used for anomalous diffraction measurements and is much lower at
liquid He temperatures (10 K) compared to 100 K were the crystallography
experiments were conducted (Fig. 2).
For future x-ray crystallography work on the Mn4Ca complex it will therefore be
imperative to develop protocols that mitigate the x-ray induced damage.
More generally, these data show that in redox-active metalloproteins careful
evaluations of the structural intactness of the active site(s) is required
before structure-function correlations can be made on the basis of high
resolution x-ray crystal structures.
Primary Citation:
References:
detailed structural features of the Mn4Ca complex during the four
intermediate steps of water oxidation have been studied extensively with X-ray
absorption, EPR, and FTIR spectroscopies. X-ray absorption spectroscopy studies
have established that the
four Mn atoms and the Ca atom in the catalytic complex are linked by
di- and mono-µ-oxo bridging atoms. The salient structural features determined
by these studies are that the Mn4Ca complex contains three Mn-Mn
distances at 2.7-2.8 Å that are characteristic of di-µ-oxo bridges, and one or
two Mn-Mn distances at 3.3 Å and two Mn-Ca distances at 3.4 Å that are
characteristic of mono-µ-oxo bridges. In addition, recent x-ray crystallography
studies of PS II have added very valuable information to our knowledge about
the structure.1-4 However, at
present there are serious discrepancies among the structural models for the
Mn4Ca complex derived from these studies,
5 and inconsistencies with X-ray,
6,7
EPR8,9 and
FTIR10-12 spectroscopic data.
This disagreement is predominantly a
consequence of x-ray-induced damage to the catalytic metal site as shown in
this study, and also differences in the interpretation of the electron density
at the presently available resolution (3.2 to 3.8 Å).
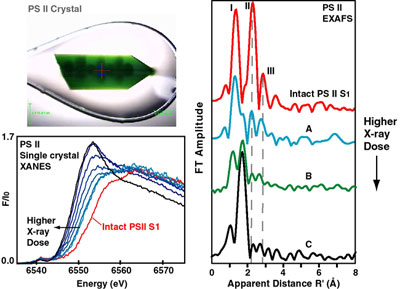
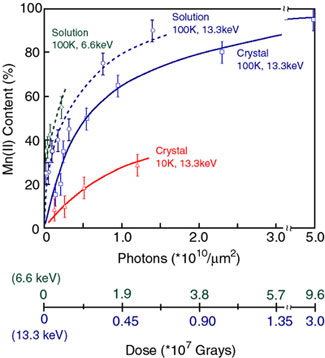
Fig. 2. Increasing Mn(II) content in PS II due to radiation damage.
(Solid blue line) Mn(II) content in PS II crystals as a function of x-ray
irradiation at 13.3 keV (0.933 Å) at 100 K. The conditions are similar to those
during x-ray diffraction data collection. The dose on the abscissa is given in
Grays and in photons/unit area, units that are commonly used for
crystallography and spectroscopy experiments, respectively. At 66% of the dose
(2.3x1010 photons/µm2) compared to
the representative average dose of (3.5x1010
photons/µm2)
used for crystallography, PS II crystals contain ~80% Mn(II). (Dashed blue
line) The damage profile for PS II solution samples is very similar to that
seen for crystals. (Dashed green line) The generation of Mn(II) is considerably
greater when the x-ray irradiation is at 6.6 keV (1.89 Å) which is the energy
at which the anomalous diffraction measurements for PS II were conducted.
(Solid blue line) The Mn(II) produced by damage in crystals is considerably
decreased when the irradiation is conducted at 10 K. This provides a method
that could be used to mitigate the effects of radiation damage during
crystallography measurements.
Yano, J.; Kern, J.; Irrgang, K.-D.; Latimer, M. J.; Bergmann, U.; Glatzel, P.;
Pushkar, Y.; Biesiadka, J.; Loll, B.; Sauer, K.; Messinger, J.; Zouni, A.;
Yachandra, V. K. X-ray Damage to the Mn4Ca Complex in Single Crystals of
Photosystem II Studied Using In Situ X-ray Spectroscopy: A Case Study for
Metallo-Protein Crystallography, Proc. Natl. Acad. Sci. USA 2005,
102, 12047-12052.