Crystal Structure of an Intermediate State of a Mechanosensitive Membrane
Channel
summary written by Raven Hanna
You have probably never seen a bacteria pop. Yet, as solution-filled balloons, bacterial cells are susceptible to changes in pressure. For example, microbes entering a fresh water solution from a salt solution would quickly succumb to death by swelling due to water rushing into the cells due to osmotic pressure differences. Bacteria do not pop because they are able to sense and respond to changes in pressure through mechanosensitive channels that transverse their membranes. These gates are like pressure relief valves, opening to ease pressure and closing when balance is restored.
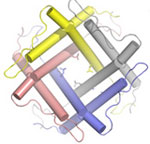
A group of researchers led by Prof. Douglas C. Rees from Caltech have solved the crystal structure of one such transmembrane gate, the mechanosensitive channel of large conductance (MscL) from Staphylococcus aureus using SSRL Beam Line 12-2. Their 3.8 Å structure shows an intermediate state of the channel, between open and closed. It can be compared to a previous crystal structure of a homologous channel that was found in the closed state. The S. aureus channel is made of four subunits, while the previous structure had five. Despite that difference, the researchers found that the architecture of the two channels is similar, including similar transmembrane helices and conserved hydrophobic residues at the point of channel constriction.
Comparisons between the geometries of the channel components of the two structures have led the researchers to propose a two-step helix pivoting model of channel gating. This work was published in the September 3 issue of the journal Nature.
To learn more about this research see the full Scientific Highlight
Zhenfeng Liu, Chris S. Gandhi and Douglas C. Rees "Structure of a tetrameric MscL in an expanded intermediate state". Nature, 461, 120-124 (2009).