A New Way to Limit Damaging Production of Nitric Oxide
summary written by Raven Hanna
Nitric oxide (NO) is one of very few gaseous signaling molecules in humans. NO causes smooth muscles to relax and blood vessels to open. Its deficiency leads to disorders such as hypertension and impotence, but too much NO can lead to rheumatoid arthritis, stroke, cancer, and other diseases. Three distinct but related enzymes (called nitric oxide synthases) make NO from an arginine molecule. One of the nitric oxide synthases, iNOS, creates localized, high concentrations of NO as part of the body's immune response. Because it is this elevated activity of iNOS that can cause disease, scientists would like to specifically inhibit the action of iNOS without interfering with the activity of the other two enzymes, eNOS and nNOS. Since the three enzymes have identical active sites (i.e. where NO is made), finding an inhibitor that will bind in this site for iNOS but not eNOS nor nNOS has proved challenging.
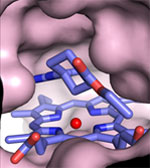
Crystal structures by a research group led by Prof. Elizabeth Getzoff of The Scripps Research Institute have revealed a mechanism that allows selective inhibition of iNOS. Using data partially collected at SSRL Beam Lines 7-1, 9-1, and 9-2, the group solved 17 structures of NOS enzymes bound to a variety of inhibitors.
Some of the inhibitors, as seen in the crystal structures, had a surprising effect on iNOS. These inhibitors bound loosely in the arginine binding area of the enzyme's active site and caused a chain reaction of structural changes. This resulted in an additional binding site opening far away from the active site. Only those inhibitor molecules that have a tail that could reach and bind in this additional site effectively inhibited iNOS but not eNOS. While the inhibitors could similarly bind at the active sites, the eNOS enzyme did not open an additional binding site for the inhibitors' tail.
Uncovering this new binding mechanism informs the future design of drugs that specifically inhibit iNOS activity. Inhibitors can have a region that binds loosely in the active site and a tail that swings around to bind in a distant, and distinct, secondary site created by the active site interactions. The researchers have called this an anchored plasticity approach and suggest that it may be useful for designing selective inhibitors for other enzymes that are difficult to target due to their similarities to other enzymes. This work was published in the November 2008 issue of Nature Chemical Biology.
To learn more about this research see the full Scientific Highlight
Garcin E.D., Arvai, A.S., Rosenfeld R.J., Kroeger, M.D., Crane, B.R., Andersson G., Andrews A., Hamley P.J., Mallinder P.R., Nicholls D.J., St-Gallay S.A., Tinker A.C., Gensmantel N.P., Mete A., Cheshire D.R., Connolly S., Stuehr D.J., Aberg A., Wallace A.V., Tainer J.A., Getzoff E.D. 'Anchored plasticity opens doors for selective inhibitor design in nitric oxide synthase.' Nat. Chem. Biol. 4 (2008), 700-707.