
Mercury is a well-known poison, but it is perhaps at its most dangerous when
bound by organic groups to form organo mercury compounds (1). Such compounds
are highly neurotoxic to mammals, but nevertheless have seen use as pesticides
and also are made by microbial meabolism of mercuric ions. Several devastating
mass-poisonings of human populations have been caused by organo mercury
compounds. Organo mercury compounds exhibit an insidious latency in the
development of toxic effects. Exposed humans may only develop toxic symptoms
after a delay of several months. Adults are affected, but exposure in
utero
results in particularly severe consequences such as microcephaly, cerebropalsy,
seizures, mental retardation, and other cruelly averse effects. Despite its
toxicity and widespread occurance, many aspects of how organomercury compounds
cause such deadly effects remains unknown.
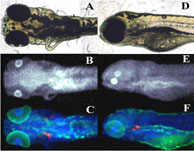 | |
Figure 1:
X-ray fluorescence images of anaesthetized zebrafish larvae. A & D optical
micrograph; B & E Hg; C & F Hg (blue), Zn (green) and Ca (red).
| |
|
In an article published in the Proceedings of the National Academy of Sciences
USA, Malgorzarta Korbas and co-workers have brought new insight into molecular
mechanisms underlying organo mercury's toxicity. The team used X-ray
fluorescence imaging conducted on SSRL beamlines 9-3 and 2-3 to study mercury
accumulation in zebrafish (Danio rerio) larvae. Newly hatched zebrafish are
relatively underdeveloped - for example, their eyes have yet to properly
develop, and they still have a yolk sac attached - and they are therefore much
used as a model organism for the study of vertebrate embryonic development and
toxicology (2). Korbas and co-workers raised newly hatched
zebrafish larvae in water containing low levels of methylmercury cysteinate
(from 200 nm to 100 μM). The team first examined whole
tricaine-anaesthetized larvae, using X-ray
fluorescence imaging to obtain the distribution maps of mercury and other
elements within the live fish. The researchers then investigated Hg
distributions in sequential sections, with alternate sections being prepared
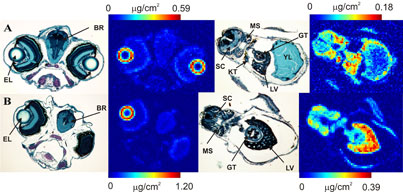
for histology and X-ray fluorescence imaging (Figure 2). Strikingly, the
greatest accumulation of methylmercury compounds was observed in the rapidly
dividing layer of the lens epithelium, visible as rings at the periphery of the
eye lenses (Figure 2,3). Lower levels of methylmercury were observed in brain,
optic nerve and various other organs (Figure 3). The data suggest that the
reported impairment of visual processes by mercury may arise not only from
previously reported neurological effects, but also from direct effects on the
ocular tissue.
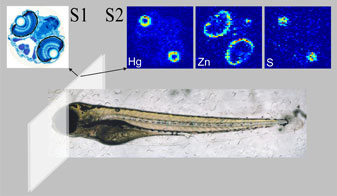 |
Figure 2:
Experimental procedure for sectioning organomercury treated zebrafish. Fish
were prepared for X-ray fluorescence imaging by using adjacent
6-μm sections,
one section mounted on a glass slide and stained for histology (S1), while the
other section, for X-ray fluorescence imaging, was placed on a plastic cover
slip (S2) with no further processing. |
|
Figure 3:
Head and liver sections from larvae treated with methylmercury cysteinate: (A)
2 μM for 36 h and (B) 200 nM for 84 h. The figure shows histological (left) and
Hg (right) images. (BR)-brain, (EL)-eye lens, (LV)-liver, (GT)-gut, (KT)-kidney
tubule, (MS)-skeletal muscle, (SC)-spinal cord.
|
 |
|
Control fish (Figure 4) showed no mercury signal, but displayed significantly
higher calcium levels in the outer layers of the retina than the mercury
exposed fish, suggesting that methylmercury might interfere with the natural
calcium distribution as has previously been suggested (3).
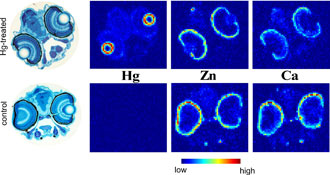 |
Figure 4:
Elemental distributions in methylmercury exposed and unexposed zebrafish.
Comparison of elemental distributions for Hg, Zn and Ca in sections of fish
heads from larvae exposed for 24 hours to 100 μM
methylmercury cysteineate
(upper) with those from control fish (lower), measured using X-ray fluorescence
imaging.
|
|
This novel approach demonstrates synchrotron X-ray fluorescence imaging of
zebrafish to be a powerful tool for investigating molecular toxicology of heavy
metals. The method is equally applicable to the study of other elements of
concern, such as arsenic, selenium, thallium and lead. The technique also
provides an ideal tool for investigating drugs such as chelation agents
(4) and
can be applied to the study of essential metals and other elements of interest
during normal development.
Primary Citation:
Korbas M, Blechinger SR, Krone PH, Pickering IJ, George GN (2008) Localizing
organomercury uptake and accumulation in zebrafish larvae at the tissue and
cellular level. Proc Natl Acad Sci USA 105:12108-12112.
References:
-
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical
compounds. Crit Rev Toxicol 36:609-662.
-
Hill AJ, Teraoka H, Heideman W, Peterson RE (2005) Zebrafish as a model
vertebrate for investigating chemical toxicity. Toxicol Sci
86:6-19.
-
Gasso S, Cristofol RM, Selema G, Rosa R, Rodriguez-Farre E (2001)
Antioxidant compounds and Ca2+ pathway blockers differentially protect against
methylmercury and mercuric chloride neurotoxicity.
J Neurosci Res 66:135-145.
-
George GN, Prince RC, Gailer J, Buttigieg GA, Denton MB, Harris HH,
Pickering IJ (2004) Mercury binding to the chelation therapy agents DMSA and
DMPS, and the rational design of custom chelators for mercury. Chem Res
Toxicol 17:999-1006.
|
|





