

The adaptive immune response enables the vertebrate immune system to recognize and respond to specific pathogens, with immunological memory allowing a stronger response upon subsequent re-exposure to a pathogen. Adaptive immunity relies on the capacity of immune cells to distinguish between the body's own cells and foreign invaders. ab T cell receptors (TCRs) recognize antigenic peptides in complex with major histocompatibility complex proteins (MHC) as the central event in the cellular adaptive immune response. Despite undergoing an extensive education process in the thymus, mature T cells exhibit a high frequency of crossreactivity, or alloreactivity, toward foreign peptide-MHC to which they have not previously been exposed. Alloreactivity indicates an inherent ability of the TCR to crossreact with a broad range of self and foreign peptide-MHC ligands, which could be beneficial for immune surveillance of a universe of potential pathogens. However, it presents a major clinical problem for organ transplantation in that genetically mismatched tissue can be rejected in a graft-host alloresponse.
The molecular basis of alloreactivity remains poorly understood, despite the
fact that the general structural principles of TCR/pMHC interactions have been
defined in approximately 14 cocrystal structures (Rudolph et al., 2006). More
broadly, receptor-ligand crossreactivity has been observed across many systems
and has generally been attributed to "molecular mimicry." However, structural
evidence for molecular mimicry, in the form of complexes between one receptor
and multiple similar, but distinct ligands, remains elusive for most systems.
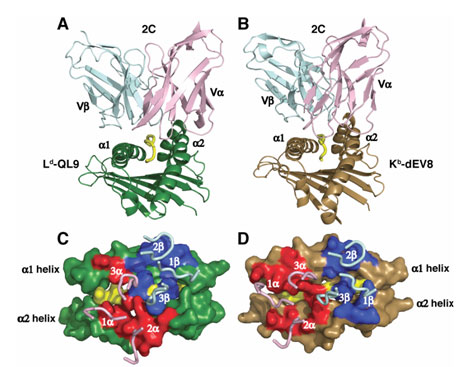
Figure 1: 2C TCR binding orientation with its self and foreign ligands.
(A,B) The a and b
chains
of the 2C TCR (pink and cyan) bind the peptide-MHC (A) foreign
(Ld-QL9, green
and yellow) and (B) self (Kb-dEV8 brown and yellow) ligands.
Only the Fv region
of the TCR and the a1 and a2 domains of the MHC are shown. (C,D) The
"footprint" view showing the isolated binding loops of the TCR over the surface
rendering of the (C) foreign and (D) self ligands. The
2C contact surface on
each peptide-MHC is drawn in red (Va) and blue
(Vb). (Colf et al., 2007)
The ability of a TCR to directly recognize foreign (allogeneic) MHC molecules
underlies T cell-mediated rejection in patients receiving allogeneic organ
transplants. In order to better understand the differences in recognition of
self versus foreign peptide-MHC complexes, we crystallized and solved the
structure of the 2C TCR bound to a foreign MHC, collecting data at SSRL
beamline 11-1. We compared our TCR/foreign MHC structure to the previously
published structure of the same TCR bound to a self MHC (Garcia et al., 1998),
revealing that instead of mimicking the interactions formed with a self MHC, a
single TCR adopts a completely different strategy to recognize a foreign MHC
(Figure 1). This is surprising, since the self and foreign MHC share 80%
sequence identity in the helices presented to the TCR. Very few conserved
interactions are found in the allogeneic and syngeneic complexes, and these
occur via largely unique binding chemistries. In addition, there does not
appear to be a focus on the polymorphic MHC residues presented to the TCR.
Thus, while sequence conservation between the self and foreign ligands might
have suggested similar recognition strategies, the interatomic contacts
highlight a vastly different mode of recognition.
In addition to the co-complex structure described, we also crystallized and
solved the structure of an engineered, high-affinity variant of the 2C TCR
bound to the same foreign MHC. In this structure, the data for which were also
collected on beamline 11-1 at SSRL, the "wild-type" recognition footprint
persists despite modified interactions with the peptide, indicating that
differences in the antigenic peptide are not necessarily sufficient to alter
TCR recognition.
Thus, we show that a single TCR recognizes two globally similar, but distinct
ligands by divergent mechanisms, indicating that receptor-ligand
crossreactivity can occur in the absence of molecular mimicry.
This work was supported by NIH grants AI 48540 (K.C.G.) and GM55767 (D.M.K.), a
National Science Foundation predoctoral fellowship (L.C.), the Keck Foundation,
and the Howard Hughes Medical Institute.
Primary Citation
References
Colf, L.A., Bankovich, A.J., Hanick, N.A., Bowerman, N.A., Jones, L.L., Kranz,
D.M., and Garcia, K.C. (2007). How a single T cell receptor recognizes both
self and foreign MHC. Cell 129, 135-146.
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |