
 A. Nikitin1),
H. Ogasawara1)
D. Mann2),
R. Denecke1)*,
Z. Zhang3),
H. Dai2),
KJ Cho3),
A. Nikitin1),
H. Ogasawara1)
D. Mann2),
R. Denecke1)*,
Z. Zhang3),
H. Dai2),
KJ Cho3),
A. Nilsson1,4)
1Stanford Synchrotron Radiation Laboratory, 2575 Sand Hill Road,
Menlo Park, CA 94025, USA
2Department of Chemistry, Stanford University, Stanford,
CA 94305, USA
3Department of Mechanical Engineering, Stanford University,
Stanford, CA 94305, USA
4FYSIKUM, Stockholm University, Albanova University Center,
S-10691 Stockholm, Sweden
In the next 20 years one of the major challenges for the modern society will be
the increasing shrinkage of available resources related to energy production
and important environmental issues associated with global warming. The
escalating growth of the population multiplied by the increase in consumption
will lead to a deficiency of basic natural resources like fossil fuels and an
increase of pollutant emission into the environment. One possible solution to
these problems is to develop entirely new technologies based on hydrogen as an
energy carrier [1]. Hydrogen can be produced from water using
either electricity generated by renewable energy sources, such as direct
photocatalysis driven by sun light and heat from nuclear reactors, and can
serve as a fuel in the fuel cells to generate electricity either stationary or
on board of vehicles.
Safe, efficient and compact hydrogen storage is a major challenge in order to
realize hydrogen powered transport. According to the DOE FreedomCAR program
roadmap the on board hydrogen storage system should provide 6 weight % of
hydrogen capacity by 2010. Currently, the storage of hydrogen in the absorbed
form is considered as the most appropriate way to solve this problem. Thus, a
media capable of absorbing and releasing large quantities of hydrogen easily
and reliably is being actively sought. Since Dillon et al.
[2] showed that carbon nanotubes can store hydrogen, this material
has been considered as a candidate for hydrogen storage media.
Physisorption and chemisorption both have been proposed as possible mechanisms
for hydrogen storage in carbon nanotubes. While most of previous studies have
focused on the hydrogen storage through physisorption, recent Density
Functional Theory (DFT) calculations for single-walled carbon nanotubes (SWCN)
[3,4] indicate the potential for up to 7.5
weight % hydrogen storage capacity for this material through chemisorption by
saturating the C-C double bonds in the nanotube walls and forming C-H bonds.
However, direct experimental evidence for hydrogen storage capacity through
chemisorption has not been demonstrated.
In this regard, the chemical interaction of hydrogen with carbon nanotubes was
studied using carbon atom specific techniques like X-ray Photoelectron
Spectroscopy (XPS) and X-ray Absorption Spectroscopy (XAS) involving the C1s
core level. These methods allow us to observe the formation of C-H bonds
through the modification of the local electronic structure around specific
carbon atoms and to quantify the amount of hydrogen that is chemically
adsorbed in terms of per carbon atom.
Using XPS as a probing tool we also studied the reversibility of the
hydrogenation of SWCN. Our results showed that SWCN film preserves its
morphology at least for two cycles of hydrogenation/dehydrogenation but the
amount of defects in the walls of SWCN increases significantly. We also find
that all C-H bonds break at the temperature above 600 °C.
The present results indicate that it is possible to form local C-H bonds by
chemical interaction between hydrogen and SWCN. To fully realize hydrogen
storage in SWCN it is essential to find means to dissociate hydrogen and to
fine tune the energetics of the C-H bonds to allow for hydrogen release at
50-100 °C. The former can be solved using an appropriate metal catalyst for
hydrogen dissociation and the latter can be accomplished by using SWCN with a
well defined radius. Theoretical calculations suggest that the C-H bond is
weaker for hydrogenated SWCN with larger radius [4].
We acknowledge Donghui Lu at beamline 5-1 at SSRL and Tolek Tyliszczak and
Hendrik Bluhm at beamline 11.0.2 at ALS for their technical support. This work
was supported by the Global Climate Energy Project and carried out at the
Stanford Synchrotron Radiation Laboratory and the Advanced Light Source,
national user facilities supported by the U.S. Department of Energy, Office of
Basic Energy Sciences.
Primary Citation
A. Nikitin,
H. Ogasawara,
D. Mann,
R. Denecke,
Z. Zhang,
H. Dai,
K. Cho, and
A. Nilsson,
References
*Permanent address: Lehrstuhl für Physikalische Chemie II, Universität
Erlangen-Nürnberg, Egerlandstr. 3, D-91058 Erlangen, Germany
For our experiments we used as grown SWCN films (Fig. 1) and in situ
hydrogenation by atomic H-beam treatment. The experiments were performed at
beamline 5-1 at the Stanford Synchrotron Radiation Laboratory (SSRL) and at
beamline 11.0.2 at the Advanced Light Source (ALS). Fig. 2 shows the C1s XPS
spectra measured for both clean and hydrogenated SWCN films. We see that H-beam
treatment leads to dramatic change in the spectral line shape of the C1s peak.
The hydrogenated SWCN exhibits a prominent shoulder between 285 eV and 286 eV
that is absent in clean SWCN film (Fig 2), leading to an increase of the peak
full width at half maximum (FWHM) from 0.44 eV to 1.3 eV. The deconvolution of
the C1s peak of hydrogenated SWCN shows good agreement in the position of peak
(2) with the theoretically predicted values of the C1s chemical shifts for C
atoms on the walls of SWCN forming C-H bonds. The assumption that peak (2) in
the C1s spectrum of hydrogenated SWCN is due to the C-H bond formation was
further supported by the C K-edge XAS spectra of the clean and hydrogenated
SWCN films (Fig. 3). In comparison with spectrum for the clean SWCN (black
curve), the hydrogenated SWCN spectrum (blue curve) shows clear decrease of
intensity in the region of p* resonance and increase
in the energy range of C-H* and s*, indicating that
H-beam treatment causes the rehybridization of the C atoms in the SWCN from
sp2
to sp3 along with the formation of C-H bonds [5].
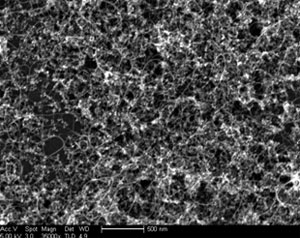
Fig. 1 SEM image of as grown SWCN film. White "ropes" are nanotubes and
nanotube bundles. White "spots" are silica catalyst support particles.
Based on the intensity ratio between peak (1) and peak (2) in the C1s XPS
spectrum, the amount of hydrogenated carbon atoms is estimated to be
65±15 %.
Under the assumption that each hydrogenated carbon atom bonds to one hydrogen
atom, we estimate the hydrogen capacity of the studied SWCN to be
5.1±1.2
weight %, which is close the above cited DOE FreedomCAR requirement of 6 weight
%. Furthermore, XPS spectra measured at different photon energies resulting in
different kinetic energies and, thus, different electron escape depths,
demonstrate that the hydrogenation takes place not only on the surface, but
also inside of the nanotube bundles.

Fig. 2
C1s XPS spectra of (a) the clean SWCN film and (b) SWCN film after
hydrogenation. Peak (1) corresponds to the signal from C atoms unaffected by
hydrogenation; whereas peak (2) is due to H coordinated C atoms. The
theoretical values of the C1s core level chemical shifts due to C-H bond
formation for different types of SWCN are shown as vertical lines. 
Fig. 3 Carbon K-edge XAS spectra of (a) clean SWCN film
and (b) SWCN film after hydrogenation.
Phys. Rev. Lett. 95, 225507 (2005)
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |