

Iron plays an integral role in many biochemical processes essential for life.
However excess iron leads to the production of highly reactive hydroxyl
radicals by Fenton chemistry (1). These free radicals are
deleterious to cells as they react indiscriminately with proteins, DNA and
lipids. Hence, iron homeostasis is a highly regulated process and is critical
to human health (2). Disorders in iron metabolism, are
however, surprisingly common. Iron deficiency affects more than one billion
people worldwide (3,4), while iron overload
disorders (hereditary hemochromatosis) are among the most frequent single gene
disorders in humans. For example occurrence of disease associated allele,
HFEC282Y, is as high as 10% in individuals of Northern European
descent (5).
In humans, 80% of the daily iron need is required for hemoglobin synthesis and
erythroid precursor cells utilize the transferrin cycle to import the needed
iron. In this cycle, 2 molecules of holo-transferrin (Tf), each loaded with 2
ferric (Fe3+) ions, bind the dimeric transferrin receptor (TfR) on
the cell surface. The Tf2:TfR2 complex is endocytosed
into the early endosome. Following acidification, Tf releases the
Fe3+, which is then reduced to Fe2+ by Steap3. Divalent
metal iron transporter 1 (DMT1) then transports the Fe2+ across the
endosomal membrane into the cytosol for use in cellular processes.
Recently, Ohgami et al. and Fleming have demonstrated that Steap3 (six
transmembrane epithelial antigen of the prostate 3) is the major erythroid
ferrireductase (6,7). Other Steap family
members are also important in human health. Steap1 and Steap2 are found at
particularly high levels in prostate cancer (10), making
Steap1 an appealing target for cancer immunotherapy (9 -12). The Steap4-/- knockout mice develop
spontaneous metabolic disease (13). Steap proteins generally
contain a C-terminal six transmembrane helical domain that co-ordinates a
transmembrane heme (7,8). Steap2-4 also
contain an oxidoreductase domain that lies on the cytosolic side of the
membrane (8), allowing them to serve as a transmembrane
reductase. Outside the Steap family, the oxidoreductase domain shows greater
sequence similarity to an archaeal protein
F420H2:NADP+ Oxidoreductase (FNO,
28% identity) than it does to other human proteins. The archaeal FNO utilizes
a 5' deazaflavin that is unknown in mammals (14). Thus, the
FNO-like domain in the mammalian Steap family is likely to use more common
flavins such as FMN or FAD. The reduction of iron is thought to occur by the
sequential, transmembrane transfer of electrons from cytosolic NADPH to
endosomal Fe3+ via the flavin and the intramembrane heme
(6-8).
The structure of Steap3 oxidoreductase domain was solved using data collected
at SSRL beamline 9-2. Crystals of Steap3 (apo-Steap3) and co-crystals of
Steap3 with NADPH (Steap3-NADPH) diffracted to a resolution of 2 Å. The phases
for the apo-Steap3 structure (PDB ID: 2vns) were determined by multiple
isomorphous replacement with anomalous scattering, using data from Pt and Hg
derivatives. The apo-Steap3 structure was used as the starting model to solve
the Steap3-NADPH structure (PDB ID: 2vq3) by molecular replacement.
These structures lacked interpretable density for residues 1 to 28 and the
N-terminal His6-tag. However, the disordered N-terminus is poorly
conserved between Steap2-4, and hence may not be essential for the
oxidoreductase activity. Since the oxidoreductase domain is N-terminal to
transmembrane domain, the C-terminus of the domain can be expected to orient
towards the membrane with the dimer axis perpendicular to membrane (Figure 1).
The oxidoreductase domain consists of 2 subdomains, the first is the classical
dinucleotide binding domain composed of 2 Rossmann folds; the second is a
C-terminal subdomain composed of 2 antiparallel b-strands with connecting
a-helices.
Comparing the substrate-free and NADPH-bound structures showed no significant
structural differences due to ligand binding. The Ca atoms in chain A of
apo-Steap3 superpose on chain A of the Steap3-NADPH structure with a root mean
square deviation (RMSD) of 0.26 Å. Both structures showed the presence of a
two fold symmetric dimer in the asymmetric unit (Figure 1).
The Steap3 core superposes on the archaeal FNO (PDB ID: 1JAX) structure with an
RMSD of 1.44 Å. However, there are critical differences, particularly with
regard to the position of the dimer interface. In Steap3, the dimer interface
is repositioned, which allows the Steap3 oxidoreductase domain to closely
approach the membrane for electron transfer to the flavin and the transmembrane
heme moiety. The NADPH is bound with the adenine ring on the membrane distal
side, and the nicotinamide moiety on the membrane proximal side, facilitating
electron transfer across the membrane (Figure 1,2A). This suggests that the
intermediate electron acceptor, presumably a flavin, will bind "above" the
nicotinamide ring, near the cytosolic face of the lipid bilayer. The
similarities between the NADPH binding site of Steap3 and that seen in FNO (PDB
ID: 1JAY) and Human Biliverdin IX Beta Reductase (BVR-B, PDB ID: 1HE4) allows
for superposition of the flavin within the Steap3 active site (Figure 2B).
However, the docking results in a clash between the conserved Leu206
and FMN (Figure 2B). Because Leu206 is the last ordered residue in
the Steap3 structure, the clash might be an artifact of the truncated protein.
The presence of the C-terminal domain might prevent the residue from crashing
down into the active site of the oxidoreductase domain. Alternatively, the
clash may suggest that a conformational change is required for the flavin to
bind, and might represent a possible gating mechanism to facilitate electron
transfer into the endosome only in response to the presence of ferric ions
(Fe3+) awaiting reduction. Binding of Steap3 to other players in
the Tf-cycle, like TfR or DMT-1, might promote the required conformational
change. Interestingly, the dimeric Tf2-TfR2 complex
carries 4 Fe3+ ions, a cargo that is nicely complimented by the
Steap3 dimer with 2 molecules of NADPH (4 electrons). Furthermore the
proximity of the binding site to the dimer interface suggests the oligomeric
state of Steap3 might also influence the binding of the flavin molecule.
Figure 2:
The Steap protein family clearly plays important roles in human health. Thus,
a deeper understanding of the structure-function relationships in these
proteins may aid the design of pharmaceuticals that specifically target
processes in which Steap proteins are involved. For example, Steap specific
inhibitors might inhibit intestinal iron uptake in iron overloaded individuals.
In this light, these studies reveal the unique structural aspects of the Steap
oxidoreductase domain, and suggest several strategies for targeting the Steap
family of metallo-reductases with high specificity, a certain requirement for
successful pharmacological intervention.
Primary Citation
References
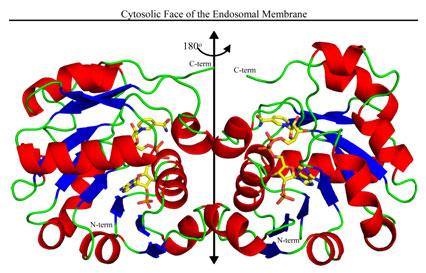
Figure 1:
The structure of the oxidoreductase dimer is depicted with helices in red,
strands in blue, connecting loops in green, and the 2-fold axis running
vertically (black double headed arrow). The truncated C-termini, which must
connect to the C-terminal transmembrane domain, are in green at the top of the
structure. NADPH (C, yellow; N, blue; O, red; and P, orange) runs up the front
side of the right subunit (back side of the left subunit) with the
adenine-ribose-2'-phosphate moieties near the bottom and the nicotinamide ring
near the top.
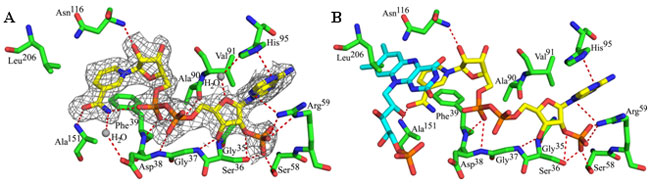
(A) The Steap3 fold and active site structure reveal a nicotinamide ring that
is bound in an unusual orientation with the amide moiety solvent exposed. The
NADPH is colored as in Fig. 1. (B) FMN from biliverdin IX-beta reductase (PDB
ID: 1HE4) is docked to the Steap3-NADPH structure by superposition of the BVR-B
nicotinamide ring onto that of the Steap3-NADPH structure. The docked FMN
clashes with the side chain of Leu206. FMN is colored similar to
NADPH, but with carbons in cyan.
Anoop K. Sendamarai, Robert S. Ohgami, Mark D. Fleming, and C. Martin Lawrence
(2008). Structure of the membrane proximal oxidoreductase domain of human
Steap3, the dominant ferrireductase of the erythroid transferrin cycle.
Proc. Natl. Acad. Sci., 105:7410-7415.
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.