

Monika Martick1,2 and William G. Scott1,3,*
The Center for the Molecular Biology of RNA1, the Department of Molecular, Cellular and Developmental Biology2, and the Department of Chemistry and Biochemistry3, Robert L. Sinsheimer Laboratories, University of California, Santa Cruz, Santa Cruz, CA 95064, USA
*Contact: wgscott AT chemistry.ucsc.edu
Since the discovery that RNA can be an enzyme, a fundamental question has emerged: How does an RNA molecule fold up into a precise three-dimensional structure capable of catalyzing a chemical reaction? This problem is interesting not only from the point of view of living organisms, but also in terms of trying to understand how a pre-biotic "RNA World" populated by ribozymes, as evolutionary precursors of today's protein enzymes found in all living organisms, might have functioned.
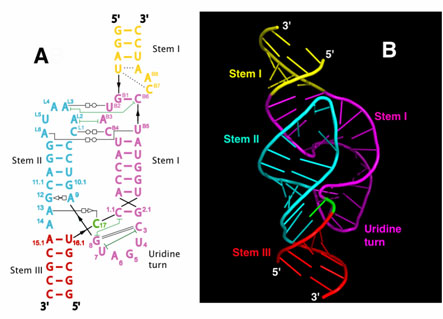 |
Figure 1 Schematic (A) and ribbon (B) diagrams depicting the crystal structure of the full-length hammerhead ribozyme. The sequence and secondary structure in Figure A is color-coded to match the structural features shown in Figure B. The cleavage site nucleotide, C-17, is shown in green, and various helical stems and loops are denoted using several other colors. Tertiary hydrogen bonding contacts are denoted as thin black lines, and tertiary stacking interactions as thin green lines. See Figure 2 for a detailed schematic representation of the active site. |
The first ribozyme structure to be elucidated was that of the hammerhead RNA, a small self-cleaving RNA1,2. The hammerhead ribozyme has been the subject of intensive investigations as it is a small, tractable archetype for studying RNA catalysis. Two crystal structures containing a minimal RNA construct that supported the self-cleaving reaction were obtained in 1994 and 1995, and appeared to be in close agreement, yet were unable to explain a growing number of biochemical experimental results.
In 2003, a more extensive (or "full-length") hammerhead RNA sequence was discovered to be 1000-fold more catalytically active3,4. To understand the basis for this catalytic enhancement, and to address the accumulating concerns regarding the earlier structures, William G. Scott's group at UCSC solved the structure of a full-length hammerhead ribozyme using X-ray diffraction data collected at Beamline 1-5 at SSRL. These latest structural results suggest how the RNA itself, rather than functioning as passive scaffold for divalent metal ions, is a direct participant in its own self-cleavage chemistry. In the new structure, invariant residues appear positioned for general base and general acid catalysis in much the same way histidines are positioned for general acid-base catalysis in RNase A (Figure 2). Hence it appears these aspects of acid-base catalysis are so fundamental that they might be considered universal principles of macromolecular enzymology (both for proteins and RNAs).
In addition to revealing the positions that several of the invariant nucleotides in the hammerhead sequence adopt in order to influence catalysis, the new hammerhead ribozyme structure reveals how an extensive tertiary contact forms between a hairpin loop capping Stem II and an unpaired region of Stem I. This contact, absent in the minimal hammerhead ribozyme structure, imparts a severe distortion upon Stem I, ultimately resulting in the distal end of Stem I becoming coaxial with Stems II and III. This tertiary contact forms much of the "top" half of the molecule as shown in Figure 1.
 |
Figure 2 A proposed mechanism for hammerhead ribozyme catalysis. The nucleotide implicated as a general base in the self-cleavage reaction, G-12, is shown in red. The nucleotide implicated in general acid catalysis, G-8, is shown in dark blue. The substrate RNA is black, and water molecules that may participate in the reaction, playing the roles of specific base and specific acid catalysts, are shown in magenta and cyan. The scissile phosphate is depicted as an (unobserved) pentacoordinated oxyphosphorane. G-12 can abstract a proton from the 2'-OH of the cleavage-site ribose only if the endocyclic nitrogen N1 becomes deprotonated (as shown). This may happen via simple ionization, or through a (rare and transient) tautomerization to the enolic form (as shown). As the 2'-proton (in yellow) is abstracted by G-12, the bond between the 2'O and the phosphorus atom forms, and that between the phosphorus and the 5'O begins to break. As the latter breaks, a negative charge accumulates on the leaving group 5'O. A proton relay may then take place in which the 5'O acquires the 2'-proton from G-8, which is simultaneously replaced with that from an adjacent water molecule or hydronium ion (as shown). |
Focusing upon the nucleotides that both structures have in common, it is apparent that a continuous deformation of the RNA can occur that transforms the previous structure into the newly observed, active structure. Using adiabatic morphing, one can compute an energetically plausible trajectory in which comparatively low-energy torsion-angle conformational changes transform the old structure into the new one in such a way that the overall fold and position of all three helical stems is approximately preserved, indicating a similar set of conformational changes may occur in solution or in crystals of the minimal hammerhead RNA. These are best viewed as a molecular movie (requires a QuickTime browser plugin).
This work was supported by
the National Institutes of Health grant AI043393 and the Center for the
Molecular Biology of RNA at University of California at Santa Cruz.
Primary Citation
Monika Martick and William
G. Scott, Tertiary Contacts Distant from the Active Site Prime a Ribozyme for
Catalysis Cell 126: 309-320 (2006).
References
Related Links
Additional Materials: http://xanana.ucsc.edu/hh/
UCSC Press Release: http://www.ucsc.edu/news_events/press_releases/text.asp?pid=907
Scott Research: http://xanana.ucsc.edu/scottlab
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |