
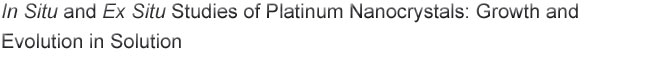
 |
Figure 1. Time-resolved XRD results obtained for both low and high concentration reactions. (A) In situ XRD plots; the position of the (111) and (200) reflections for bulk fcc platinum is shown by the lines at bottom; (B) time evolution of area under the Pt(111) peak. I - IV denote the growth stages of the high concentration reaction; (C) evolution of X-ray correlation length (L) calculated from FWHM of Pt(111) peak. |
In this study in situ and ex situ techniques were used to
investigate the growth of platinum nanocrystals in solution. Synchrotron-based
XRD was employed to study the growth of platinum nanocrystals in real time and
to provide a direct assessment of the crystallinity, nanocrystal concentration,
and crystallite size of the product. TEM was then used to investigate the
nanoparticle morphology at different growth stages.
The synthesis involved thermal decomposition of a platinum precursor in a
reaction cell in the presence of a stabilizing agent, under a hydrogen
atmosphere. For the in situ XRD, the reaction cell was made of stainless
steel and measurements were conducted at the Stanford Synchrotron Radiation
Lightsource (SSRL) at beam line 7-2. Two different concentrations, referred to
as low and high concentrations were investigated. For each experiment, about
0.05 mL of the precursor solution was injected to the reaction vessel. The cell
was flushed through with H2, before being filled to 200 kPa
H2 and heated to 70°C, which was the temperature maintained for
the duration of the experiment. Products collected from the experiments were
purified and the samples studied in the TEM.
In situ X-ray diffraction
Figure 2.
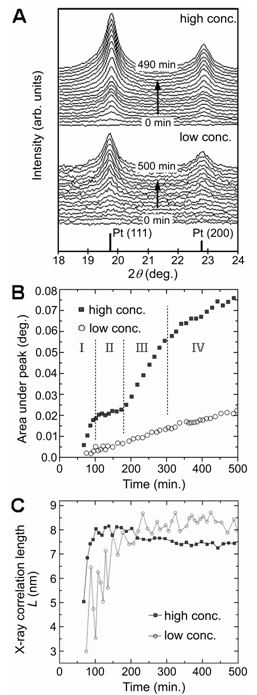
TEM images of platinum nanoparticles obtained from high concentration reactions
of (A, B) 75, (C, D) 120, (E, F) 240, and (G, H) 500 minutes.
For the high concentration reaction, the peak area plot is more complex and can
be divided into four stages based on the slope: Stage I: ~60 100 min.; Stage
II: 100 180 min.; Stage III: 180 300 min.; Stage IV: 300 500 min. The peak area
increases rapidly and linearly in Stage I, indicating a ten fold increase in
the growth rate of [PtNC] compared to the low concentration reaction.
Remarkably, during Stage II, the peak area remains almost constant, indicating
almost no growth in [PtNC]. In Stage III, the peak area resumes a
rapid increase; the gradient is ~60% of that observed in Stage I. From the
beginning of Stage IV to the end of experiment, the slope of the area plot
decreases slightly, with a gradient of about 1/3 of that in the previous stage
(Stage III), indicative of a slower increase in [PtNC].
The X-ray correlation length (L) for the low concentration reaction
increases from 3 nm at 75 minutes to 8.6 nm at 230 minutes. It then remains
almost constant at ~8.4 ± 0.3 nm for the remainder of the reaction. For
the high concentration reaction, L increases during Stage I from ~5 nm
to ~8.2 nm at 100 minutes. During Stage II, L remains almost constant at
8.0 ± 0.2 nm. Interestingly during Stage III, L decreases from 8.1
nm to 7.6 nm, differing markedly from the low concentration reaction. After 300
minutes,
L continues to decrease gradually and is calculated to be ~7.4 nm at the end of
Stage IV.
Transmission electron microscopy
Figures 2A, C, E, and G show the TEM images of platinum nanoparticles collected
from the high concentration reaction at 75, 120, 240, and 500 minutes,
corresponding to Stages I to IV, respectively. As shown in Figure 2A, the
platinum nanocrystals formed in the middle of Stage I adopt a nearly cubic
morphology with eight arms/pods, described as quasi-octapods. Figure 2C
shows the platinum nanocrystals collected in the middle of Stage II are larger
and have thinner cross-sections towards the center of the {100} faces,
indicative of etching. For this reason we describe these nanocrystals as
etched-octapods. Figure 2E and G shows the platinum nanocrystals formed in
Stage III and are described as porous platinum or porous nanocrystals.
Growth mechanism: Low concentration.
Growth mechanism: High concentration reaction.
Stage II: Quasi-octapods → etched-octapods. There is little
change in the total amount of platinum atoms in the nanocrystals
([PtNC]) in Stage II. TEM images show growth on the
quasi-octapods
takes place over the arms along the <111> directions, while etching has
occurred on the {100} facets, leading to the formation of
etched-octapods. By
correlating the observations in XRD and TEM, the increase in [PtNC]
due to nanocrystal growth is balanced by the loss of re-dissolved platinum
atoms from the nanocrystals as a consequence of etching; hence the
[PtNC] peak area remains constant over this growth stage.
Stage III: Etched-octapods → porous nanocrystals. The relatively
rapid increase in [PtNC] in Stage III is similar to that observed in
Stage I, again indicating kinetic control. However, the slight decrease in
L suggests an unconventional type of growth. The cross-sections towards
the center of the (100) faces of the porous nanocrystals at 240 minutes
are noticeably thinner (Figure 2F) as compared with those of the
etched-octapods
(Figure 2D). A significant change in the particle morphology is gradually
taking place, which is characterized by etching of much of the central core and
the formation of more branches. These changes lead to an increase of porosity
in the nanocrystals. As the nanocrystals become increasingly porous, the
average size of the single crystalline domains, and hence L, decreases.
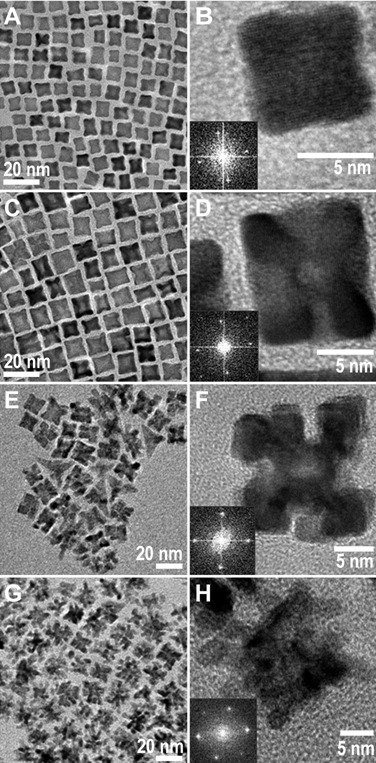
Figure 3 illustrates the proposed growth mechanism from Stage I to III.
Figure 3.
Schematic illustration of the growth mechanism for platinum nanocrystals in the
high concentration reaction, through the first three growth stages (I - III).
Platinum monomers first nucleate into cuboctahedral nuclei (A), and then grow
into single-crystalline quasi-octapods (B). Growth of the arms of the
quasi-octapods, coupled with selective etching on the edges and centers of
facets leads to formation of etched-octapods (C). These processes
continue and transform the nanocrystals to porous nanocrystals (D).
These findings demonstrate the utility and complementarity of TEM and
in situ XRD for revealing the growth mechanism of nanocrystals of
different morphologies. Furthermore, it is hoped that the understanding
developed will aid in the design of new synthetic methodologies, which are
applicable to other fcc metals, as shown by our application to palladium.1
Primary Citation
Cheong, S., Watt, J., Ingham, B., Toney, M. F. & Tilley, R. D. In situ
and ex situ studies of platinum nanocrystals: growth and evolution in
solution.
J. Am. Chem. Soc. 131, 14590-14595 (2009).
References
In situ XRD plots for both low and high concentration experiments are
shown in
Figure 1A. As the reactions proceed, small peaks corresponding to Pt(111) and
Pt(200) begin to emerge and progressively grow over time. The initial peaks are
generally broad indicating the formation of small crystallites. As the reaction
proceeds the peak areas increase indicating an increase in the concentration of
nanocrystalline platinum ([PtNC]) and the peaks get narrower
indicating an increase in the average crystallite size.
Figure 1B shows the plot of area under the Pt(111) peak against time. The peak
area plot for the low concentration reaction increases approximately linearly
over the course of experiment, indicating a continuous and almost linear
increase of [PtNC].
TEM images of platinum nanoparticles obtained from the low concentration
reaction show faceted nanoparticles of irregular shapes, with particle sizes of
6 ± 2 nm. The size range is consistent with the X-ray correlation length
shown in Figure 1C. TEM images of the platinum nanoparticles collected after
400 minutes display similar morphologies to those observed at 80 mins but are
of bigger size at 11 ± 3 nm, again consistent with XRD.
The peak area plot in Figure 1B shows the [PtNC] growth rate is much
slower for the low concentration reaction, compared to the high concentration
reaction. TEM indicates the formation of faceted and non-branched morphologies
from an early growth stage. Slow nanocrystal growth and formation of faceted,
non-branched morphologies are known to be characteristic of growth in a
thermodynamically controlled regime. Nanocrystal growth in the low
concentration reaction therefore occurs under thermodynamic control via a slow,
nearly layer-by-layer monomer addition onto the crystallite faces to yield
stable, faceted morphologies.
Stage I: Formation of quasi-octapods. The XRD data show that the
increase of [PtNC] during Stage I is rapid: there is fast
nanocrystal growth. A fast growth rate has been associated with kinetically
controlled growth, which often leads to a complex growth pattern and
nanocrystals having branched quasi-octapods morphologies.
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.