

T.-C. Weng, & J.E. Penner-Hahn, University of Michigan
J.S. Magyar & H.A. Godwin, Northwestern University
Lead poisoning can damage the brain and nervous system and is particularly
dangerous for young children who are still developing. It is estimated that
~2.2% of all U.S. children aged 1-5 years (434,000 children) have elevated
blood lead levels (BLLs) (i.e., µ10 g/dL), and in certain communities this
number is as great as 15%. The developmental toxicity associated with childhood
lead poisoning has been attributed to interactions of Pb(II) with proteins
containing thiol-rich structural zinc-binding sites. Recently, Penner-Hahn,
Godwin and co-workers have used x-ray absorption spectroscopy to define the
local structure of the Pb(II) ion in such sites, providing critical insights
into how lead alters the structure of these proteins [J. S. Magyar et
al., J. Am. Chem. Soc. 127, 9495-9505].
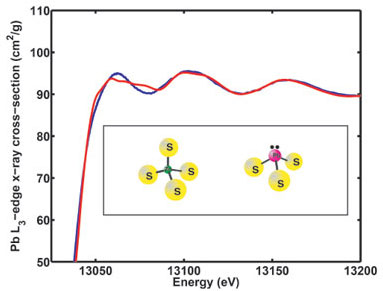
The results of this study show that when Pb(II) is bound to structural
zinc-binding peptides it binds in a three-coordinate Pb(II)-S3
mode, in contrast with Zn(II), which is known to bind in a four-coordinate mode
in these proteins. This Pb(II)-S3 coordination in peptides is
consistent with a trigonal pyramidal Pb(II)-S3 model compound
previously reported by Bridgewater and Parkin, but it differs from many other
reports in the small molecule literature which have suggested
Pb(II)-S4 as a preferred coordination mode for lead. Reexamination
of the published structures of these "Pb(II)-S4" compounds reveals
that, in almost all cases, the complexes are actually oligomers with effective
Pb coordination numbers of 5, 6, or 8. The results reported herein combined
with this new review of published structures suggest that lead prefers to avoid
four coordination in sulfur-rich sites, binding instead as trigonal pyramidal
Pb(II)-S3 or as Pb(II)-S5-8.
These data demonstrate that the Pb(II) coordination sphere is significantly
different from that of Zn(II) bound to the same peptides. Zinc binding to
CP-CCCC is tetrahedral, with Zn-S4 coordination; Zn(II) binding in
CP-CCCH, CP-CCHC, and HIV-CCHC is also tetrahedral but with
Zn-S3N coordination. By
contrast, Pb(II) in both CP-CCCC and CP-CCCH is three-coordinate,
Pb-S3.
Because tetrahedral zinc coordination is essential for proper folding of these
peptides, these results provide a simple explanation for the observation that
Pb(II) does not induce proper folding of the peptides, even when it binds more
tightly than zinc. Structural zinc-binding domains are commonly found in
transcription factors and proteins involved in gene expression. In these
proteins, the zinc-binding domain is the part of the protein that directly
binds to DNA; improper folding of these domains will reduce or prevent
protein-DNA binding. The studies reported here provide the first detailed,
molecular insights into how lead binding to these proteins could directly
account for some of the severe developmental problems known to result from lead
poisoning.
Primary Citation:
J. S. Magyar, T.-C. Weng, C. M. Stern, D. F. Dye, B. W. Rous, J. C. Payne, B.
M. Bridgewater, A. Mijovilovich, G. Parkin, J. M. Zaleski, J. E. Penner-Hahn
and H. A. Godwin, "Reexamination of Lead(II) Coordination Preferences in
Sulfur-Rich Sites: Implications for a Critical Mechanism of Lead Poisoning",
J. Am. Chem. Soc. 127, 9495 (2005)
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |