

 |
|
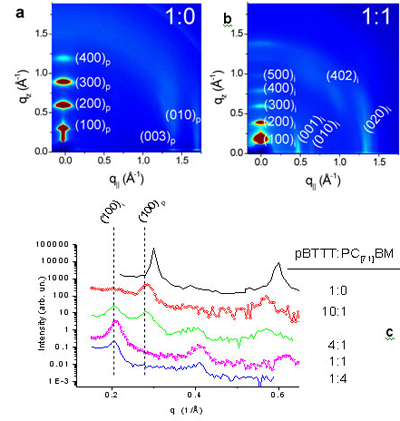
| Figure 1: Diffraction data for pBTTT:PC[71]BM blends of varying weight ratio that have been annealed at 185 °C for 10 minutes. The annealing took place at the glass transition temperature of pBTTT in order to increase the molecular order. (a) 2D GIXS of pure pBTTT. (b) 2D GIXS of 1:1 pBTTT:PC[71]BM blend. (c) High resolution specular x-ray diffraction for a series of pBTTT:PC[71]BM blends. This confirms the expansion perpendicular to the substrate. | |
Stanford and SLAC researchers have recently investigated a highly crystalline
polymer that reaches optimal solar cell efficiency at 80% fullerene content.
X-ray diffraction measurements (see figure 1) performed at the Stanford
Synchrotron Radiation Lightsource (SSRL) have shown the existence of a highly
ordered bimolecular crystal (a structure wherein two distinct chemical species
arrange in an ordered fashion on a lattice). This crystal forms when the
fullerene molecule intercalates between the side-chains of the semi-crystalline
polymer (see figure 2). While such co-crystals of fullerenes and small organic
molecules have been observed before, this is, to our knowledge, the first
report of a fullerene-polymer co-crystal. Furthermore, this unexpected
intercalation is observed in several polymer:fullerene blends and suggested by
the researchers for several others. These results explain the origin of the
optimal 1:4 blending ratio in these blends: this ratio yields a two phase film
that consists of the bimolecular crystal and a pure fullerene phase with
approximately equal volumes, while for a blend ratio of ~1:1 only the pure
bimolecular crystal forms.
The research team has specifically studied thin film BHJs of
poly(2,5-bis(3-tetradecyllthiophen-2-yl)thieno[3,2-b]thiophene) (pBTTT) and
either phenyl-c61-butyric acid methyl ester (PC[61]BM) or
phenyl-c71-butyric acid methyl ester (PC[71]BM). pBTTT is a
well-ordered semicrystalline polymer that has high charge carrier mobility (see
SSRL highlight on Highly
Oriented Crystals in Polythiophenes for details). The 1:1 pBTTT:PCBM blends
had a solar cell efficiency of only 0.16%, while 1:4 blends had an efficiency
of 2.35%. X-ray diffraction measurements on SSRL beam lines 7-2, 2-1 and 11-3
were conducted to understand these unexpected results. Typical data are shown
in Fig. 1 for pure pBTTT and a series of pBTTT:PC[71]BM blends of
varying weight ratio. These specular scans (fig. 1c) demonstrate the shift in
the lattice perpendicular to the substrate as PC[71]BM is added. The
specular x-ray pattern for the pristine pBTTT film (black line) shows that the
d-spacing is 21.15 Å. The scan for the 4:1 blend (green line) shows the
presence of a shifted peak for the 10:1 blend (shifted due to defects in the
PBTTT), but most importantly, a new peak emerges with an expanded d-spacing of
30.2 Å. With the addition of more fullerene, the peak corresponding to
the intercalated lattice increases in intensity and the 22.1 Å peak
associated is completely suppressed at a blending ratio of 1:1. The existence
of two sets of peaks shows that the fullerenes are not distributed uniformly
throughout the film, but form distinct phases: pure pBTTT and the
pBTTT:PC[71]BM intercalated phase. We use the subscript "p" to refer
to the pristine pBTTT lattice, while the intercalated phase peaks are noted
with the subscript 'i'.
Additional diffraction data were obtained using 2D grazing incidence x-ray
scattering (2D GIXS) for several pBTTT:PC[71]BM blends. The 2D GIXS
for a pristine pBTTT film (Fig. 1a) is consistent with the literature and
displays several diffraction peaks along the vertical slice at q||
≈ 0. These peaks are (100)p, (200)p, etc. and
correspond to a lamellar d-spacing of 21.15 Å, consistent with the higher
resolution data in Fig 1c. The scattering for pristine pBTTT also exhibits two
vertical streaks at q|| equal to 1.41 Å-1 and 1.71
Å-1 corresponding to the intramolecular (003)p and
the pi-stacking (010)p, respectively. The 2D GIXS pattern for a 1:1
pBTTT:PC[71]BM blend, shown in Fig. 1b, is significantly different
from the pattern for the pristine film in that the peaks along the vertical
slice at q|| ≈ 0 are shifted and now correspond to the
d-spacing of 30.2 Å. There are several new streaks that appear along
q||
with the strongest at 0.49 and 0.67 Å-1 and a broad, mixed
index peak at q|| = 1 Å-1 and qz = 0.8
Å-1. The 2D GIXS pattern for the 1:4 pBTTT:PC[71]BM
blend contains these peaks which we associate with the intercalated lattice as
well as a halo around 1.4 Å-1. This halo corresponds to a pure
amorphous PC[71]BM phase that arises when phase separation occurs.
The absence
of the fullerene halo in the 2D GIXS pattern for the 1:1 blend is additional
evidence for the fullerene being intercalated between the polymer side-chains.
We attribute the ~9 Å increase in the d-spacing normal to the substrate
(21.1 to 30.2 Å) to the intercalation of the PC[71]BM in
between the side-chains of the pBTTT. A space-filling schematic of pBTTT and
PC[71]BM drawn to scale is shown in Fig 2(b) and demonstrates how
the side-chain spacing along the polymer backbone allows for this. A
preliminary unit cell is assigned based on the diffraction data: a triclinic
lattice with a = 30 Å, b = 9.9 Å, c = 13.5 Å, a = 72°, B ≈
90°, and
g ≈ 90°. This is in contrast to the
reported orthorhombic pBTTT lattice of a = 22.15 Å, b= 3.67 Å, and
c = 13.37 Å (although it appears the lattice is actually triclinic with a
= 19.6 Å, b = 5.4 Å, c = 13.6 Å, a =
136°, B =
84° and g = 86°). The
preliminary bimolecular lattice preserves the intramolecular (c axis) spacing,
but there is a doubling along the b-axis due to the presence of the PCBM;
hence, there are two polymer molecules along the b direction. A more detailed
structural analysis is in progress at SSRL.
For all the polymers studied by the Stanford/SLAC research team, they found
that if there was ample room between the polymer side-chains, then
polymer:fullerene intercalation occurred. It is, however, not yet clear if
intercalation is desirable for solar cell devices. It will be necessary to
adjust the position of fullerenes and other electron acceptors with different
shapes and sizes to determine if intercalation is beneficial for solar energy
conversion. None-the-less, our findings suggest that separate models of
recombination should be developed for the two different kinds of cells that
form depending on whether or not intercalation occurs.
Primary Citation
"Bimolecular crystals of fullerenes in conjugated polymers and the implications
of molecular mixing for solar cells", A.C. Mayer, M.F. Toney, S.R. Scully, J.
Rivnay, C.J. Brabec, M. Scharber, M. Koppe, M. Heeney, I. McCulloch, M.D.
McGehee, Adv. Func. Mater. 19, 1173-1179 (2009).
This research shows that the rational design of polymers for BHJ solar cells
must consider the possibility of bimolecular crystal formation in efforts to
push the efficiency higher. These discoveries further suggest a method of
intentionally designing bimolecular crystals and tuning their properties to
create novel materials for photovoltaic and other applications such as LEDs,
LASERs and biosenors.
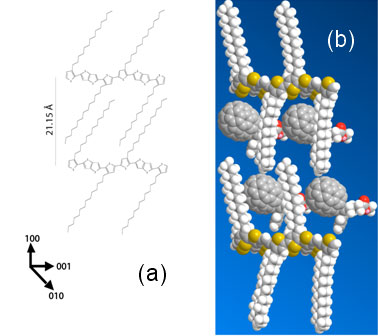
Figure 2:
Possible bicrystal structure showing the effect of PC[71]BM
intercalation on the crystal lattice of pBTTT. (a) pristine pBTTT (b)
Schematic of intercalated pBTTT:PC[71]BM. The
PC[71]BM is placed within the
intercalated pBTTT:PC[71]BM in order to agree with the d-spacings found in
x-ray scattering. The lattice axes are shown in the lower left corner for
reference.
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.