

Most eukaryotic viruses, including HIV, influenza and herpes viruses, undergo
maturation when transitioning from the noninfectious provirion to the
infectious virion. Maturation processes involve reorganization of viral
quaternary structure to defend viral gene from the cellular defense mechanism
and lead to effective transfection. Nudaurelia capensis omega virus,
NwV, is a T=4, non-enveloped, icosahedral, single
strand RNA virus, where T is the triangulation number defining an icosahedral
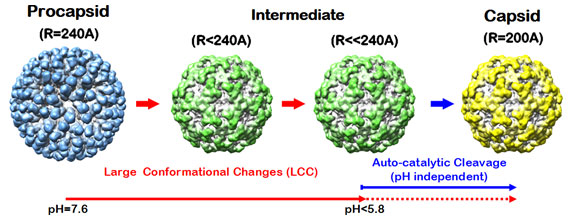
lattice of the virus capsid structure. Virus like particles (VLPs) of NwV exhibit large pH-dependent conformational changes (LCC)
when the procapsid, purified at pH=7.6, (~480 Å) is exposed to pH=5.0,
resulting in ~400 Å particles (Figure 1). In response to the LCC, an
auto-proteolysis occurs in which each of 240 subunits is cleaved at
Asn570-Phe571 (1). We investigated this pH-induced
maturation by equilibrium and time-resolved small angle X-ray scattering (SAXS)
at SSRL beam line 4-2.
We showed that when the acidic interfaces of the NwV
subunits are protonated the electrostatic repulsion between adjacent subunits
is weakened, allowing the particle to undergo spontaneous size reduction
through a LCC. This condensation was studied at different pH values allowing
the determination of a titration curve that demonstrated a continuous change in
particle size with an overall particle pKa = ~5.8. An N570T mutation of the
NwV subunits, that does not undergo the maturation
cleavage, shows identical behavior to the wild type at pH values between 7.6
and 6.0. However, they exhibit different maturation properties at pH values
between 5.8 and 5.0, with the mutant (uncleaved) particle displaying
systematically larger radii above pH 5.0.
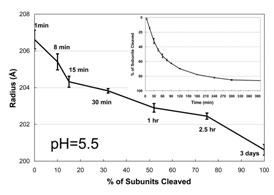
To directly determine the relationship between cleavage and particle size,
time resolved SAXS studies were carried out at pH 5.5 (the maximum pH at which
100% cleavage occurs in 3 days) (Figure 2). At pH=5.5, auto-proteolysis was
required to achieve the final mature size, whereas particles below pH 5.0 did
not require cleavage to achieve the final mature size.
The particle radius decreases from 236 Å to 207 Å in one minute and
then slowly decreases in size to ~200 Å as shown. The size change is
closely proportional
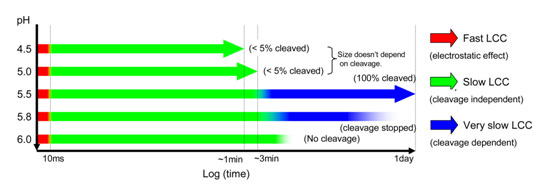
Finally, fast time resolved studies were performed at different pH values
(Figure 3). The time resolution for the experiment is ~10 ms, and data frames
were recorded successively after the drop in pH using a stopped-flow mixing
apparatus. The results demonstrated 3 kinetic stages in the particle
condensation with each incremental drop in pH, the first completing within 10
ms, the second in less than 5 seconds and the third in the 2-3 minute time
regime corresponding to the annealing described above. In addition to those
stages, the slow cleavage dependent stage (hours) is required at pH values
between 5.8 and 5.5 (Figure 2). Those maturation events at the quaternary
structure level are reminiscent of protein folding, where there is rapid
formation of a molten globule, followed by different stages of polypeptide and
side-chain annealing.
The study breaks new ground in understanding the energy landscape associated
with virus maturation, a process common in complex human viruses, and required
for a provirion to become an infectious virion. Encoded within the provirion
structure is a program that strengthens the capsid and activates an
auto-catalytic cleavage of the subunits.
Primary Citation
Matsui, T., Tsuruta, H. & Johnson, J.E. Balanced Electrostatic and Structural
Forces Guide the Large Conformational Change Associated with Maturation of T=4
Virus. Biophys. J., 98, 1337-1343 (2010).
References
to the fraction of subunits cleaved, emphasizing the role of cleavage at this
pH in the final stages of particle condensation. The kinetics of particle
cleavage at pH 5.5 is shown in the Figure 2 inset (1). The results indicated
that structural forces prior to cleavage counter the reduction in electrostatic
force and that the structural resistance due to the repulsive intersubunit
interaction is reduced when the auto-proteolysis occurs. These studies also
show that there is a significant period of protein annealing required for the
capsid to reach its equilibrium dimension. Based on the crystallographic
structure of the fully mature virion, we hypothesize that the annealing
involves positioning of the molecular switches associated with the T=4
quasi-symmetry as well as the formation of the active site for
auto-proteolysis.
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.