
Autism is a neurodevelopmental disorder that impairs social interactions, and
causes communication deficits and repetitive behaviors. About 1 in every 150
children is affected by autism. Genetic screens revealed that mutations in the
neurexin and neuroligin genes are among the multiple genetic causes of autism
spectrum disorders and mental retardation (Jamain et al., 2003;
Szatmari et
al., 2007). In the brain, neurexins and neuroligins are cell adhesion
proteins on the pre-synaptic and post-synaptic cell membranes, respectively.
Their extracellular domains interact with each other within the synaptic cleft
to provide connectivity between nerve cells and assure proper synapse function.
Neuroligins are involved in maturation of synapses by validating excitatory
versus inhibitory synapses (Chubykin et al., 2007). Mice lacking
neuroligin or neurexin genes show improper synapse function and are not
viable although synapse formation itself is not affected (Missler et
al., 2003; Varoqueaux et
al., 2006). Understanding the molecular mechanism of these proteins in
synapse development is a first step towards development of novel therapeutics
directed to treat and possibly cure autism. However, up to now, the
lack of a high-resolution structure of the neuroligin/neurexin complex has
hindered studies of the function of these proteins.
In this study, we determined the high-resolution three-dimensional structures
of neuroligin-1 in isolation and in a complex with neurexin-1b by X-ray crystallography using data collected at SSRL
Beamline 11-1 and ALS Beamline 8.2.2. Neuroligin 1 is responsible for
validating excitatory synapses (Chubykin et al., 2007). Our structure
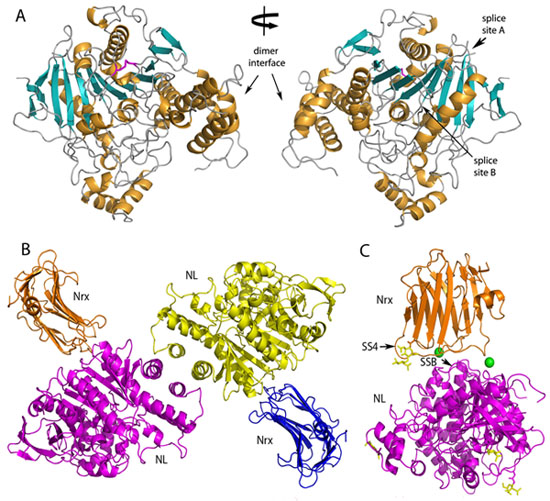
of the extracellular domain of neuroligin-1 has an ellipsoid shape and forms a
constitutive dimer (Figure 1A). The dimer interface comprises a four-helix
bundle composed of two helices from each molecule. Our structure of the
neuroligin-1 bound to neurexin-1b reveals that two
neurexin-1b molecules bind to two identical surfaces
on the opposite faces of the neuroligin-1 dimer to form a heterotetramer
(Figure 1B). The neuroligin-1/neurexin-1b complex
exhibits high affinity, and includes a large binding interface that contains
bound Ca2+. Alternatively spliced sites in neurexin-1b and in neuroligin-1 are positioned nearby the binding
interface and regulate the strength of the interaction (Figure 1C).
|  | |
|
Figure 1 Structures of Neuroligin-1 and the Neuroligin-1/Neurexin-1b complex.
A) Ribbon representation of a neuroligin-1 monomer. Two views are shown related
by a 180° rotation around the specified axis. a-helices are colored orange and
b-sheets are colored cyan.
B) Ribbon representation of the neuroligin-1/neurexin-1b heterotetramer. C) Overall view of a
neuroligin-1/neurexin-1b heterodimer showing
carbohydrates (yellow sticks), splice sites SS4 of neurexin-1b and SSB of neuroligin-1 (arrows) and Ca2+
ions at the binding interface (green spheres).
| |
|
Our structures suggest a model of the structural organization of these
cell-adhesion proteins at the synaptic junction. The arrangement of the
neuroligin-1/neurexin-1b complex positions the
C-terminal stalk regions of neuroligin-1 and
neurexin-1b at opposite faces of the
heterotetramer, thus the
complex bridges the 15-20 nm wide synaptic cleft by tethering to the pre- and
post-synaptic membranes through the stalk regions of neuroligin-1 and
neurexin-1b (Figure 2).
We used the structure of the complex to determine residues that are critical
for the interaction of neuroligin-1 with neurexin-1b. Our mutations reduced the
affinity of neuroligin-1 for neurexin-1b up to
three orders of magnitude and confirmed the binding interface revealed by the
neuroligin-1/neurexin-1b complex structure. In
future experiments, such mutants can be used as molecular
tools to study the role of neuroligins and neurexins in synapse validation. Our
results provide molecular insights for understanding the role of the
neuroligin/neurexin interaction in synapse function.
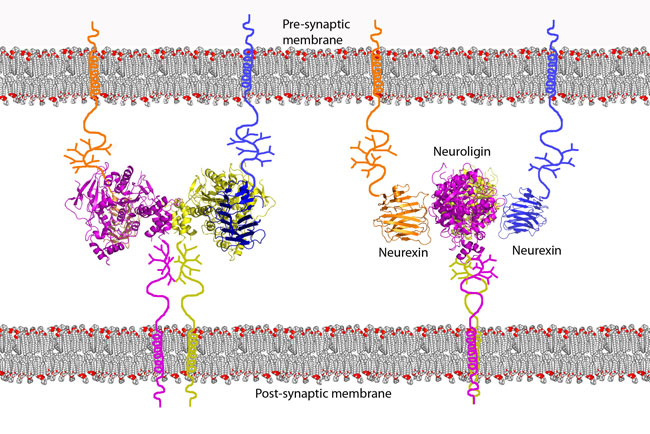 |
Figure 2 Model for the arrangement of the neuroligin-1/neurexin-1b complex at
the synapse. Neurexins are tethered to the pre-synaptic cell membrane and
neuroligins are tethered to the post-synaptic cell membrane by their stalk
regions. Their interaction forms trans-synaptic connectivity.
|
Primary Citation
D. Araç, A.A. Boucard, E. Özkan, P. Strop, E. Newell, T.C. Südhof, A.T.
Brunger. (2007) Structures of Neuroligin-1 and the
Neuroligin-1/Neurexin-1b
complex reveal specific protein-protein and protein-Ca2+
interactions. Neuron, 56, 992-1003.
References
|
Chubykin, A.A., Atasoy, D., Etherton, M.R., Brose, N., Kavalali, E.T., Gibson,
J.R., and Sudhof, T.C. (2007). Activity-dependent validation of excitatory
versus inhibitory synapses by neuroligin-1 versus neuroligin-2. Neuron
54, 919-931.
Jamain, S., Quach, H., Betancur, C., Rastam, M., Colineaux, C., Gillberg, I.C.,
Soderstrom, H., Giros, B., Leboyer, M., Gillberg, C., et al. (2003). Mutations
of the X-linked genes encoding neuroligins NLGN3 and NLGN4 are associated with
autism. Nat Genet 34, 27-29.
Missler, M., Zhang, W., Rohlmann, A., Kattenstroth, G., Hammer, R.E., Gottmann,
K., and Sudhof, T.C. (2003). Alpha-neurexins couple Ca2+ channels to synaptic
vesicle exocytosis. Nature 423, 939-948.
Szatmari, P., Paterson, A.D., Zwaigenbaum, L., Roberts, W., Brian, J., Liu,
X.Q., Vincent, J.B., Skaug, J.L., Thompson, A.P., Senman, L., et al.
(2007). Mapping autism risk loci using genetic linkage and chromosomal rearrangements.
Nat Genet 39, 319-328.
Varoqueaux, F., Aramuni, G., Rawson, R.L., Mohrmann, R., Missler, M., Gottmann,
K., Zhang, W., Sudhof, T.C., and Brose, N. (2006). Neuroligins determine
synapse maturation and function. Neuron 51, 741-754.
|
| PDF
Version | | Lay Summary | |
Highlights Archive
|
| SSRL is supported
by the Department of Energy, Office of Basic Energy Sciences. The SSRL
Structural Molecular Biology Program is supported by the Department of Energy,
Office of Biological and Environmental Research, and by the National Institutes
of Health, National Center for Research Resources, Biomedical Technology
Program, and the National Institute of General Medical Sciences. |
|



