 Samuel M. Webb (Stanford Synchrotron Radiation Laboratory), Bradley M. Tebo
(Oregon Health and Science University), and John Bargar (Stanford Synchrotron
Radiation Laboratory).
Samuel M. Webb (Stanford Synchrotron Radiation Laboratory), Bradley M. Tebo
(Oregon Health and Science University), and John Bargar (Stanford Synchrotron
Radiation Laboratory).
|  |
|
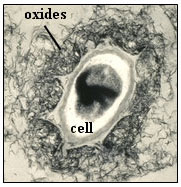
Figure 1. Manganese oxides precipitated around a spore (cell)
of the marine Mn(II)-oxidizing bacterium, Bacillus sp., strain SG-1.
This cell is about 0.5 µm diameter (small axis). |
Manganese oxides are formed in soils, watersheds, and sea water via bacterial
catalysis of the oxidation of dissolved Mn(II) to Mn(IV) (1).
These remarkable but poorly understood oxides are relatively abundant in the
environment (Mn is the second most abundant trace metal in the earths crust),
where they are among the most aggressive scavengers of metal ions
(1-3), and thus act as important sources and
sinks for heavy metals. As a result Mn biooxides can exert a large influence
over the trace metal chemistry of natural waters. For example, in
acid-mine-drainage impacted streams, bacteriogenic Mn oxides commonly form
coatings on cobbles and mineral grains in the stream and can naturally remove
large amounts of heavy metal contaminants such as zinc from the water (4). Major scientific questions that come to
the fore with respect to this issue are, "How (by what physical and chemical
mechanisms) do Mn biooxides take up such high concentrations of metals?", and
"How can we harness these processes to enhance the clean-up of
metal-contaminated waters (in engineered remediation technologies, for
example)?"
Uranium is a key contaminant of concern at US DOE sites and shuttered mining
and ore processing locations around the united sites. Migration of uranium in
ground water has contaminated drinking supplies in some locations (5,6) and threatens water supplies in
other locations, leading to the need for costly clean-up activities. A major
challenge to remediating ground water is that the contamination often resides
at significant depth in the subsurface and is spread out over very large areas
(hundreds of square meters to square kilometers). Subsurface remediation
technologies, which are often designed to immobilize the metal contaminant of
concern in place in the aquifer, therefore must utilize naturally existing
reactants to produce products that are stable in the environment. Mn biooxides
are of interest in this context because they may co-occur, or be stimulated to
grow, in subsurface areas contaminated with uranium. However, the atomic-scale
mechanisms by which they sequester uranium have received little attention.
In this study, the methods by which bacteriogenic Mn oxides sequester
hexavalent uranium, U(VI), were investigated by a collaborative group of
scientists from SSRL and the Oregon Health and Science University (7). Two complementary synchrotron based techniques
were used to study these materials under conditions similar to those which may
occur in the field: EXAFS (extended x-ray absorption fine structure)
spectroscopy, which probes the short-range atomic structure (up to ~6 Å) within
the materials. In-situ x-ray diffraction was used to probe long-range
atomic order, including particle size and crystallinity.
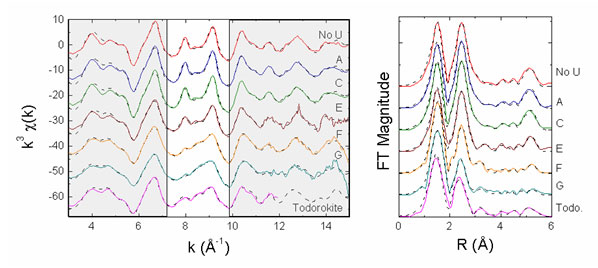
Mn K-edge EXAFS data are shown in Figure 2, where they are compared to the
spectra for bacteriogenic Mn oxides ("No U" spectrum), which exhibit layered
Mn-oxide structures, and the spectrum for a 3x3 tunnel-structure Mn oxide,
todorokite ("Todo"). As can be seen in this figure, incorporation of U(VI) into
Mn biooxides during oxide formation leads to a structure that is locally like
todorokite, especially at the highest concentrations used. Fits to the spectra
are consistent with the presence of a todorokite-like Mn oxide. At lowest U(VI)
concentrations, fits to the spectra indicate a layered Mn oxide structure.
|  |
|
Figure 2.
Mn K-edge EXAFS data and Fourier Transforms. Data are in the solid lines,
EXAFS fit in the dotted lines. Spectra A-G correspond to increasing U(VI)
concentration from 50 nM to 20 µM (spanning a range of U(VI) concentrations
representative of contaminated ground water). Oxides were grown in these
solutions using 20 µM Mn(II) and spores of the Mn-oxidizing bacterium
Bacillus
sp., strain SG-1 at pH 7.7. Clear area emphasizes parts of spectra where
systematic changes can be seen.
|
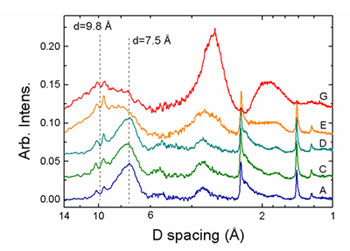
In-situ XRD results are shown in Figure 3. In samples A-D the patterns exhibit
a broad 7.5 Å basal plane reflection and in-layer 2.45 and 1.40 Å peaks, as
expected for layered Mn oxides. The breadth and low intensity of the basal
peaks are typical of very small particle size as well as dispersion of the c
axis repeat distance. The two small sharp peaks at 9.4 and 10.1 Å are due to
diffraction from the bacterial cells. As the concentration of U(VI) increases
(samples E and G), the intensity of the phyllomanganate basal plane reflection
decreases and a new broad peak at 9.8 Å is observed, indicating the presence of
a phase with long-range structure distinct from the layered Mn oxides. The
shift from 7.5 Å to 9.8 Å occurs at sample E, which is the same region where
EXAFS indicated that significant changes in the Mn local structure occurred.
Spacings of 9.8 Å are exhibited by several manganates, including hydrated and
expanded phyllomanganates as well as 3x3 tunnel structures, such as todorokite.
This result is consistent with the Mn K-edge EXAFS result in that it suggests
that the initial phyllomanganate Mn structure (low U(VI) concentration) was
altered by U(VI) incorporation. XRD patterns from samples E and G also exhibit
significant diffuse scattering located at 3.25 and 1.9 Å, which becomes more
intense as U increases. Although it is difficult to assign structures that
|  | |
|
Figure 3. Diffraction data from selected samples. Scattering from the
oxides becomes more diffuse as U(VI) concentration increases. The two sharp
peaks around a 10 Å d-spacing are structure from the bacterial cells.
| | | |
correlate to such a large, diffuse region of scattering intensity, the
locations of the broad peaks are similar to reflections expected for
todorokite. Diffraction simulations of todorokite with U(VI) present in the
tunnels show that higher order peaks are enhanced over the intensity of the
(001) reflection. Therefore, it is reasonable that these diffuse scattering
peaks relate to the incorporation of U(VI) into the structure. The breadth of
these peaks indicates a high degree of disorder, likely dominated by the fact
that these particles may be aggregates of nanoparticles. Using the Scherer
equation as a first-order approximation, a particle size estimate of 1.2 nm is
obtained for sample G, suggesting that the cross-linked tunnel-like layers are
essentially only one unit tall.
Fits to U LIII-edge EXAFS spectra (7) indicate that at
lowest U(VI) concentrations, U(VI) is bonded to the surfaces and edges of the
Mn oxides. At highest U(VI) concentrations, U(VI) is mostly bonded within
tunnels in the Mn oxide structure. Altogether, these results indicate that, in
the presence of U(VI), a transformation of the bacteriogenic Mn oxides occurs,
with U(VI) being structurally bound within the resulting pseudo-tunnel nano-Mn
oxide structures (Figure 4).
|  |
|
Figure 4.
Schematic representation of the effect of U(VI) on the structure of
bacteriogenic Mn oxides. At low U(VI) concentrations, the oxides have layered
structures (left hand side). As U(VI) concentration increases, the structure
becomes increasingly disordered, eventually exhibiting tunnels, in which U(VI)
is bound.
|
| |
These findings are significant in the context of uranium transport in aquifers
because they show that U(VI) may be structurally bound within bacteriogenic Mn
oxides. Structural binding mechanisms provide a relatively high capacity to
sorb the contaminant and simultaneously allow for slow release kinetics as
compared to other modes of binding such as sorption at particle surfaces. These
results indicate that bacteriogenic Mn oxides may be suitable for the long-term
stabilization of subsurface U(VI) contamination in in-situ engineered
remediation technologies.
This work was support by the National Science Foundation, Chemistry Division
and by the US DOE, Office of Biological and Environmental Research,
Environmental Remediation Sciences Program. This research was carried out at
the Stanford Synchrotron Radiation Laboratory, a national user facility
operated by Stanford University on behalf of the U.S. DOE, Office of Basic
Energy Sciences. The SSRL Structural Molecular Biology Program is supported by
the Department of Energy, Office of Biological and Environmental Research, and
by the National Institutes of Health, National Center for Research Resources,
Biomedical Technology Program.
References
-
Tebo B. M., Bargar J. R., Clement B., Dick G., Murray K. J., Parker D., Verity
R., and Webb S. (2004) Manganese biooxides: properties and mechanisms of
formation. Annual Review of Earth and Planetary Science 32,
287-328.
-
Villalobos M., Bargar J. R., and Sposito G. (2005a) Mechanisms of Pb(II)
sorption on a biogenic maganese oxide. Environmental Science and
Technology 39, 569-576.
-
Villalobos M., Bargar J. R., and Sposito G. (2005b) Trace metal retention on
biogenic manganese oxide nanoparticles. Elements 1, 223-226.
-
Fuller C. C. and Harvey J. W. (2000) Reactive uptake of trace metals in the
hyporheic zone of a mining-contaminated stream, Pinal Creek, Arizona.
Environmental Science and Technology 34, 1150-1155.
-
Board F. C. A. (1995) Fernald Citizens Task Force: Recommendations on
Remediation Levels, Waste Disposition, Priorities, and Future Use. US.
Department of Energy, Office of Environmental Management.
-
Morris D. E., Allen P. G., Berg J. M., Chisholm-Brause C. J., Conradson S. D.,
Donohoe R. J., Hess N. J., Musgrave J. A., and Tait C. D. (1996) Speciation of
uranium in Fernald soils by molecular spectroscopic methods: characterization
of untreated soils. Environ. Sci. Technol. 30, 2322 -2331.
-
Webb S. M., Fuller C. C., Tebo B. M., and Bargar J. R. (2006) Determination of
uranyl incorporation into biogenic manganese oxides using x-ray absorption
spectroscopy and scattering. Environmental Science and Technology
40, 771-777.
|
| PDF
Version | | Lay Summary | |
Highlights Archive
|
| SSRL is supported
by the Department of Energy, Office of Basic Energy Sciences. The SSRL
Structural Molecular Biology Program is supported by the Department of Energy,
Office of Biological and Environmental Research, and by the National Institutes
of Health, National Center for Research Resources, Biomedical Technology
Program, and the National Institute of General Medical Sciences. |
|

 Samuel M. Webb (Stanford Synchrotron Radiation Laboratory), Bradley M. Tebo
(Oregon Health and Science University), and John Bargar (Stanford Synchrotron
Radiation Laboratory).
Samuel M. Webb (Stanford Synchrotron Radiation Laboratory), Bradley M. Tebo
(Oregon Health and Science University), and John Bargar (Stanford Synchrotron
Radiation Laboratory).



