
John R. Bargar1, Samuel M. Webb2, and Bradley M. Tebo2
1Stanford Synchrotron Radiation Laboratory
2Oregon Health and Sciences University
 |
|
|
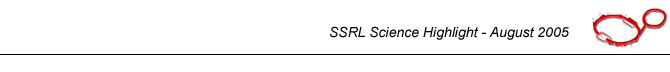
Figure 1. Top: Half of the Earth's annual photosynthetic CO2 fixation budget is attributable to oceanic phytoplankton. Mangan-ese required for this photo-synthetic activity is derived largely from bacteriogenic man-ganese oxides. Bottom: man-ganese oxides precipitated around a spore (cell) of the marine Mn(II)-oxidizing bac-terium, Bacillus sp., strain SG-1. This cell is about 0.5 µm diameter (small axis). | |
Bacterial oxidation of Mn(II) impacts the global geochemical cycling of carbon,
nitrogen, sulfur, nutrients, and contaminants in the environment. Manganese is
abundant in the biosphere (~1014 Kg of suspended and dissolved
manganese in the oceans) and is second only to iron in relative terrestrial
abundance of transition metals. Manganese is an important nutrient in the
marine water column and is fundamentally required for photosynthesis. The
acquisition of manganese by organisms and the biogeochemistry of manganese in
the oceans is therefore an essential part of global carbon fixation processes
(i.e., uptake and conversion of CO2 to organic molecules,
biomass). Manganese oxides are formed in sea water via bacterial catalysis of
the oxidation of dissolved Mn(II) to Mn(IV) (Tebo et al.,
2004). The oxides form external to bacterial cells (on cell surfaces or
bacterial exopolymers) and subsequently settle through the marine water column,
where they are available to react with dissolved ions. Bacteriogenic manganese
oxides have high surface areas, are among the strongest sorbents of heavy
metals, and are powerful oxidants of organic materials. Via sorption and
oxidation/reduction reactions, settling manganese oxides help to mediate the
trace element and nutrient composition of sea water. One of the most
fundamental of scientific questions relating to this subject is, "What are the
structures and compositions (i.e., the identities) of marine bacteriogenic
manganese oxides?". Identifying marine manganese oxides will substantially
enhance our ability to model and understand their roles in maintaining the
chemistry of the oceans. This information will also directly contribute to a
greater understanding of the properties of bacteriogenic manganese oxides,
which are of great interest for their potential technological applications.
A collaborative group of scientists from SSRL and the Oregon Health and Science
University have used the in-situ synchrotron-based techniques, EXAFS
(extended x-ray absorption fine structure) spectroscopy and XRD (x-ray
diffraction), to determine the identities of manganese oxides formed in sea
water by the marine bacterium, Bacillus sp., strain SG-1. Both
techniques provide information regarding the molecular-scale structures
(i.e., the arrangement of atoms) of the bacteriogenic oxides. These
techniques are highly complementary; EXAFS is primarily sensitive to variations
in the local structure (up to about 6 Å) around manganese atoms
and can be measured from noncrystalline substances such as amorphous colloids
and species dissolved in solution. In comparison, XRD is highly sensitive to
the long-range structure (up to about 20 Å), disorder, and particle size in
crystalline materials averaged over the entire unit cell
(i.e., the fundamental
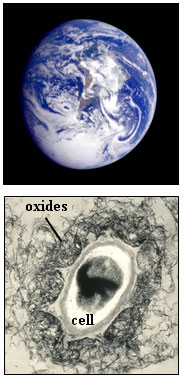
Figure 2. (A) X-ray diffraction intensity data for bacteriogenic
manganese
oxides produced in sea water. The bottom pattern is for clean cells. (B) X-ray
diffraction intensity data for synthetic birnessite reference compounds. From
Webb et al. (2005a).
X-ray diffraction data for bacteriogenic manganese oxides grown in sea water
are shown in Figure 2(a) (Webb et al., 2005a). The data
for the samples reacted for ≥ 24 hr are similar to the x-ray diffraction
pattern for the layered Mn(IV) oxide, c-disordered hexagonal birnessite (figure
2(b)); Most importantly, they exhibit strongly asymmetric (200)/(110) peaks (at
ca 1.4 Å) and (310)/(020) peaks (ca 2.4 Å). This result
qualitatively suggests
that the bacteriogenic oxides are layered manganese oxides. The layer repeat
distance of the bacteriogenic oxides is 10 Å, as indicated by the position of
the (001) peak. Close inspection of the sample patterns reveals small peaks
that indicate the presence of a related layered manganese oxide, triclinic
birnessite. In particular, the presence of two (310)/(020) diffraction peaks
and a small peak on the left-hand side of the (200)/(110) peak indicate that
the birnessite layer unit cell has undergone a slight transformation (a slight
lengthening along one of the unit cell axes), causing a breakdown of the
initial hexagonal symmetry (the designation "triclinic" indicates the lowest
possible unit cell symmetry). The x-ray diffraction pattern for the 12 hr
sample shows only hexagonal birnessite peaks, suggesting that it is the initial
phase. Note that the peaks are very weak and broad for this sample, indicating
very poor crystallinity of the material. If bacteriogenic manganese oxides are
grown in a NaCl solution, which is devoid of Ca2+, Mg2+
and other important
ions in sea water, then the resulting primary bacteriogenic oxides remain very
poorly crystalline (no diffraction peaks), and hexagonal birnessite is not
produced over this time scale (Bargar et al., 2005; Webb
et al., 2005b). This
comparison suggests that one of the major ions in sea water is required to
generate or stabilize these marine birnessites. Subsequent measurements have
shown this ion to be Ca2+, which is incorporated in the interlayer of the oxide
structure (Webb et al., 2005a).
Figure 3. EXAFS spectra for bacteriogenic manganese oxides produced in
sea water. Solid lines indicate experimental data and dashed lines indicate fits.
(a-e) bacteriogenic manganese oxides: (a) t=6h, (b) t=12h, (c) t=24h, (d)
t=50h, (e) t=80h. (f-i) reference compounds: (f) d-MnO2, (g) hexagonal
birnessite, (h) triclinic birnessite, (i) todorokite. From Webb
et al., (2005a).
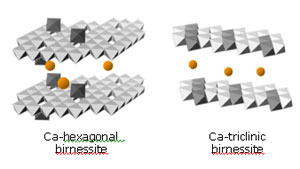
Figure 4.
Polyhedral illustration of the proposed bacteriogenic manganese oxides produced
in sea water. Octahedra denote the positions of MnO6 groups (Mn atoms in the
center, oxygen atoms at the corners). Light-colored octahedra are Mn(IV),
dark-colored octahedra are Mn(III). Orange spheres show interlayer
Ca2+
cations. Mn(III) cations are partially ordered into rows in the triclinic
birnessite structure, which breaks the hexagonal layer symmetry of the
hexagonal birnessite.
The conclusion from both sets of measurements is that the bacteriogenic
manganese oxide produced in sea water is a poorly crystalline layered manganese
oxide, birnessite. No other forms of manganese oxide were observed. One
important process that this result helps to illuminate is the photocatalyzed
reductive dissolution of manganese oxides in sea water (i.e.,
sunlight-driven reduction of Mn(IV) to Mn(II), followed by release of Mn(II)).
This reaction occurs in the surface mixed layers of the oceans, where it helps
to maintain a pronounced manganese concentration maximum (Sunda
and Huntsman, 1988). Birnessite is particularly susceptible to
photo-stimulated reductive dissolution (Sherman, 2005). Thus,
the dominance of birnessite as the primary bacteriogenic marine manganese oxide
would help to explain this chemical behavior in the surface layers of the
oceans.
This work was support by the National Science Foundation, Chemistry Division
and Earth Sciences Division. This research was carried out at the Stanford
Synchrotron Radiation Laboratory, a national user facility operated by Stanford
University on behalf of the U.S. Department of Energy, Office of Basic Energy
Sciences. The SSRL Structural Molecular Biology Program is supported by the
Department of Energy, Office of Biological and Environmental Research, and by
the National Institutes of Health, National Center for Research Resources,
Biomedical Technology Program.
Primary Citation:
References:
crystal structure repeat unit). Both techniques can be used to measure wet,
undisturbed samples, which is critical for capturing the chemistry that occurs
under natural wet conditions.
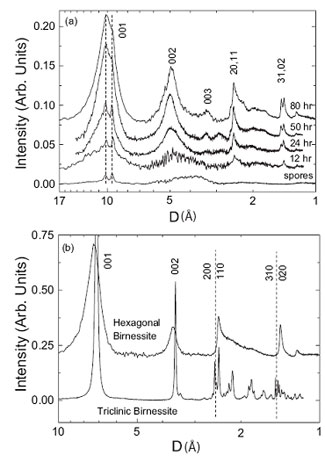
EXAFS spectra for bacteriogenic manganese oxides grown in sea water are shown
in Figure 3. Visual inspection of figure 3 shows that the sample spectra ((a) -
(e)) match the spectra for hexagonal birnessite (spectrum (g)) and
s-MnO2
(spectrum (f)), which is a disordered form of hexagonal birnessite. Fits to the
EXAFS spectra indicate the initial bacteriogenic product to be a layered
hexagonal birnessite and provide the positions of atoms within the manganese
oxide layer. EXAFS fits further indicate that the structure contains numerous
Mn(IV) vacancy defects (~ 14% of all manganese sites are empty), which
contribute substantially to the chemical reactivity of this material. With
time, a small fraction of triclinic birnessite appears. Integration of the
EXAFS and XRD results allows the structures of the bacteriogenic oxides to be
reconstructed, as shown in figure 4.
S. M. Webb, B. M. Tebo, and J. R. Bargar, "Structural Characterization of
Biogenic Mn Oxides Produced in Seawater by the Marine Bacillus sp. Strain
SG-1", Am. Mineral. 90,
1342 (2005)