

Owen W. Duckworth (North Carolina State University), John R. Bargar (Stanford Synchrotron Radiation Lightsource), and Garrison Sposito (University of California-Berkeley)
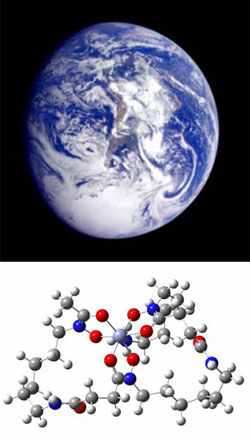 | ||
| Figure 1. Top: Iron is thought to limit phytoplankton in much of the world's oceans. Bottom: Structure of the iron-siderophore complex ferrioxamine B [Fe(III)HDFOB+]. Image courtesy of Andrzej Jarzecki, Brooklyn College, the City University of New York. | ||
Iron is one of several essential nutrients thought to limit phytoplankton
growth in large areas of the world's oceans. The growth of marine phytoplankton
represents a important linkage in the carbon cycle, accounting for
approximately 50% of the total biological uptake of carbon dioxide (Field et
al., 1998); thus, phytoplankton growth may be important for carbon
sequestration as the global carbon dioxide concentration rises. In the marine
environment, iron is typically found in subnanomolar total concentrations
(0.02-1 nM), which presents major challences to microorganisms that require Fe
for growth and photosynthesis in the oceanic water column. One strategy
utilized by marine bacteria is the production of siderophores, high-affinity
organic chelating agents they exude to increase the solubility and uptake of
iron. The bulk of soluble iron is contained within organic complexes (Donat and
Bruland, 1995), and siderophores are belived to be a major component of the
organic ligands bound to Fe in ocean waters (Witter et al., 2000). Processes
that disrupt the siderophore-mediated solubilization and biological uptake of
iron are therefore relevant to global carbon cycling. This work focuses on a
little-explored but potentially very significant disruptive pathway, namely the
direct scavenging of siderophore-Fe complexes by layer type manganese oxides.
These minerals, which are abundant in the marine water column (0.035-0.87 nM)
(Landing and Bruland, 1980), have been called the "scavengers of the sea"
because of their high affinity for trace metals (Goldberg, 1954). A major
unanswered question is "Does the Mn cycle disrupt siderophore-based Fe
acquisition?" Because of the essentiality of iron to microbial growth, this
phenomenon may represent a heretofore unknown interferring role that marine Mn
oxides may play in the mediation of iron uptake and bioavailability in the
oceans.
A collaborative group of scientists from SSRL, University of
California-Berkeley, and North Carolina State University have coupled wet
chemical measurements with EXAFS (extended X-ray absorption fine structure)
spectroscopy, to examine the molecular-scale mechanisms that could disrupt
siderophore-based Fe acquisition. In particular, research was performed to
determine the extent of sorption (bonding of the complexes to the surfaces of
manganese oxides) and the molecular architecture of surface-bound
iron-siderophore complexes (Figure 1) on manganese oxides (Duckworth et al.,
2008). Because of its ability to probe the local arrangement of atoms
specifically around iron atoms, EXAFS spectroscopy is uniquely suited to this
problem by providing information regarding the molecular-scale structures of
iron's local coordination environment. Furthermore, this technique allows for
in situ measurements of wet, undisturbed samples, which is crucial to obtaining
results that are representative of the aqueous environment.
We studied the sorption reaction of ferrioxamine B [Fe(III)HDFOB+,
an Fe(III) chelate of the trihydroxamate siderophore desferrioxamine B (DFOB)]
with two synthetic birnessites [layer type Mn(IV) oxides] and a biogenic
birnessite produced by Pseudomonas putida. We found that all of these
predominantly Mn(IV) oxides scavenged Fe(III)HDFOB+ complexes, thus
greatly reducing their aqueous concentration at pH 8. To study the molecular
nature of the interaction between the Fe(III)HDFOB+ complex and the
oxide surface, Fe K-edge EXAFS spectroscopy was employed. Visual inspection of
the EXAFS data (Figure 2) reveals that samples containing siderophore complexes
sorbed to Mn oxides (Figure 2B-E) closely resemble a Fe-doped Mn oxide standard
(Figure 2F) instead of an aqueous siderophore complex standard (Figure 2A).
This observation suggests Fe(III) associated with the Mn(IV) oxides is not
complexed by DFOB, as in solution, but instead Fe(III) is directly adsorbed to
the mineral structure. Structural modeling of the EXAFS spectra reveals that
most Fe(III) sorbs to the surface at the edges of particles and at point defect
sites (Figure 3), with there being no evidence of DFOB complexation, thus
indicating that the Mn(IV) oxides actually displaced Fe(III) from the
siderophore complex.
The ability of manganese oxides to remove Fe from a common siderophore is a
remarkable finding because Fe(III)HDFOB+ complexes are highly
stable, requiring enzymatic intervention to extract the bound ferric iron. The
common occurrence of manganese oxides in the oceans suggests that the sorption
of complexed iron to oxide surfaces could compete with siderophores-mediated
transport and uptake of iron. In high-nutrient low-chlorophyll regions of the
ocean, iron is typically a limiting nutrient (Behernfeld and Kolber, 1999), and
the presence of manganese oxides could thus impact primary productivity on a
global scale. Furthermore, Mn oxides are common in freshwater and soil
environments (Post, 1999), suggesting that this process may also be important
in terrestrial environments. Future work will help understand the effects of
siderophore structure on the stability and reactivity of metal-siderophores
complexes in natural waters.
This work was funded by the National Science Foundation, Collaborative Research
Activities in Environmental Molecular Science (CRAEMS) program (CHE-0089208).
Support was also provided by the SSRL environmental remediation sciences
program. This research was carried out at the Stanford Synchrotron Radiation
Lightsource, a national user facility operated by Stanford University on behalf
of the U.S. DOE, Office of Basic Energy Sciences. The SSRL Structural Molecular
Biology Program is supported by the Department of Energy, Office of Biological
and Environmental Research, and by the National Institutes of Health, National
Center for Research Resources, Biomedical Technology Program.
Primary Citation:
References:
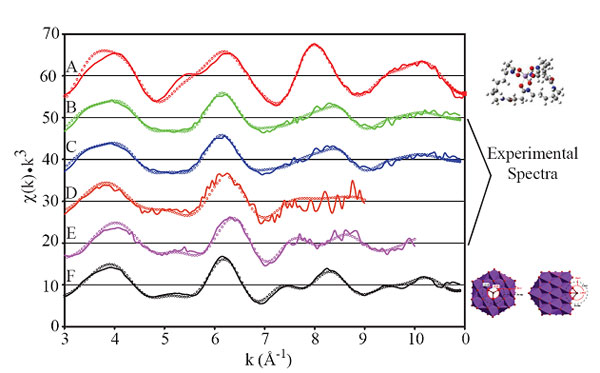
Figure 2.
Fe K-edge EXAFS spectra of Fe standards and ferrioxamine B reacted with layer
type manganese oxides. (A) 50 mM Fe(III)HDFOB+(aq); (B, C)
Fe(III)HDFOB+ reacted with biogenic manganese oxide produced by the
bacterium, P. pudita; (C, D) Fe(III)HDFOB+ reacted with
synthetic Mn(IV) oxides and (F) 20% Fe-doped d-MnO2. Lines are experimental data and open
circles are fits based on structural modeling. Spectral and fitting data are
from Duckworth et al., 2008.
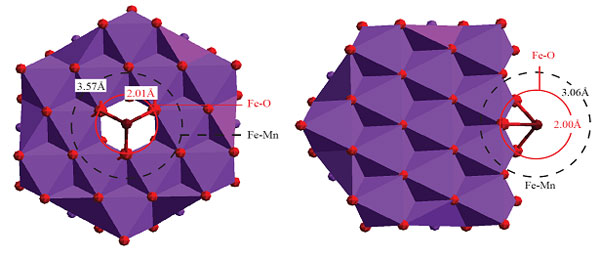
Figure 3.
Sorption structures of Fe associated with Mn(IV) oxides.
Top: Sorption of an
iron atom above a vacancy defect as a surface complex. Bottom: Sorption
of an iron atom to a particle edge as a surface complex. In all renderings, a
single layer of a manganese oxide is shown. Legend: red = oxygen; purple =
manganese; crimson = iron; gray and black circles and represent radii of
constant Fe-O and Fe-Mn interatomic distances, respectively. Lines do no
necessary pass through all atoms in a shell because some atoms may be on the
opposite side of the layer. Oxygen atoms coordinating Fe but not bonded to Mn
oxide are omitted for clarity.
Duckworth, O.W., Bargar, J.R. and Sposito, G., 2008. Sorption of ferric iron
from ferrioxamine B to synthetic and biogenic layer type manganese oxides.
Geochim. Cosmochim. Acta, 72: 3371-3380.
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.