
 Magnus Sandström,1 Farideh Jalilehvand,2 Emiliana
Damian,1 Yvonne Fors,1 Ulrik Gelius,3
Mark Jones,4 and Murielle Salomé5
Magnus Sandström,1 Farideh Jalilehvand,2 Emiliana
Damian,1 Yvonne Fors,1 Ulrik Gelius,3
Mark Jones,4 and Murielle Salomé5
1Structural Chemistry, Stockholm University, Sweden
2Department of Chemistry, University of Calgary, Alberta, Canada
3Department of Physics, Uppsala University, Sweden
4The Mary Rose Trust, HM Naval Base, Portsmouth, UK
5European Synchrotron Radiation Facility (ESRF), Grenoble,
France

Figure 1.The starboard side of the Mary Rose (about ½ of the hull, ~280 tons oak timbers) is since 1994 being sprayed with an aqueous solution of PEG 200. | |
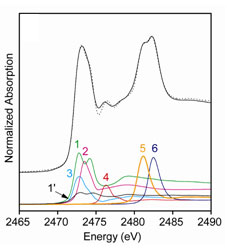
Figure 2. Sulfur K-edge XANES spectrum of Mary Rose oak core surface
(0-3 mm). Standard spectra used for model fitting: 1 (solution),
1' (solid)
disulfides R-SS-R (cystine with peaks at 2472.7 and 2474.4 eV); 45%; 2:
Thiols R-SH (cysteine, 2473.6 eV) 23%; 3: Elemental sulfur
(S8 in xylene
2473.0 eV) 10%; 4: Sulfoxide R(SO)R' (methionine sulfoxide, 2476.4 eV)
5%; 5:
Sulfonate R-SO3-
(methyl sulfonate, 2481.2 eV) 10%;
6: Sulfate SO42- (sodium
sulfate, 2482.6 eV) 7%. |
The Mary Rose was for 35 years a principal warship in Henry VIII's navy before she suddenly capsized and sank in 1545, maneuvering for battle with a French fleet outside Portsmouth, U.K. The starboard side of the wreck (Figure 1) was salvaged in 1982. Spray treatment with an aqueous solution of polyethylene glycol (PEG), to replace water and prevent cracking when drying the waterlogged wood, started in 1994 (Jones 2003). Recently, x-ray absorption near edge structure (XANES) spectroscopy was used at SSRL to show that about 2 tons of sulfur in reduced forms slowly are oxidizing to sulfuric acid in the almost intact hull of the 17th century Swedish warship Vasa (Sandström, et al., 2002). The concern for this unique historical artifact has led to the international "Preserve the Vasa" project, started in late 2003. Methods are being developed to remove or stabilize the sulfur and iron compounds, and to neutralize the acid that may continue to form in marine archaeological wood after finishing the standard PEG conservation treatment. For a better understanding of the sulfur accumulation and acid-forming processes, investigations were also started of the Mary Rose timbers, which were preserved under somewhat different conditions (Jones 2003, Sandström et al., 2005).
The total sulfur and iron concentrations were determined by x-ray photoelectron
spectroscopy, high resolution x-ray fluorescence line scans and elemental
analyses in several oak wood cores (0.4×15 cm) sampled from Mary
Rose timbers (Sandström et al., 2005). Along all
cores the sulfur concentration fluctuates around 1 mass% S, indicating totally
about two tons sulfur in the hull. This rather uniform sulfur distribution
throughout the Mary
Rose timbers differs from that in the Vasa, with high accumulation
of sulfur and iron only in the surface layers (~1 cm), degraded by bacteria.
Sulfur K-edge XANES spectra, measured at the SSRL beamline 6-2, were used to
determine in situ sulfur species with characteristic functional groups
in segments along the cores. Principal component analyses indicated at least 6
significant sulfur components. Their relative amounts were evaluated by curve
fitting with normalized XANES spectra of known "standard" compounds, mostly in
solution (Figure 2).
In most cases several types of reduced species contribute to the major peak at
2473 eV: thiols (R-SH), disulfides (R-SS-R', with a characteristic shoulder at
2474.4 eV), elemental sulfur (S8), and occasionally also pyrite
(FeS2). Magazine stored timber and surface samples show minor amounts of
sulfonates (R-SO3-) and sulfate
(SO42-), while hull timber under spray
treatment has almost no sulfate. Sulfoxides R(SO)R', with a peak at about 2476
eV, can be discerned as a minor component, a few %, in all XANES spectra.
The near anoxic environment at or below the seafloor that slows down wood
degradation, at the same time promotes the formation of the reduced sulfur
Figure 3. Scanning x-ray micro-spectroscopy (SXM) images of
Mary Rose
wood to map reduced and oxidized sulfur species over a sample area at high
spatial resolution, 0.5 µm, and spectral resolution, 0.5 eV), (brighter
color → higher concentration): (top) oak core at 2473 eV, sampled
from hull timber under spray treatment. Micro-XANES curves (not shown)
indicate that the bright spot 1 is a pyrrhotite (Fe1-xS)
particle, and spot 2 corresponds to thiols in the middle lamella.
After salvage, with access of atmospheric oxygen to the moist wood,
acid-producing oxidation of the reduced sulfur compounds becomes a conservation
concern, especially in the presence of catalytically active iron(II) ions. To
determine the distribution of the reactive sulfur species we examined thin wood
slices, cut perpendicular to the cell walls, by scanning x-ray absorption
spectro-microscopy (SXM) at beamline ID21 of the European Synchrotron Radiation
Facility (ESRF). Raster scanning of the samples was performed in the focused
beam at energies of characteristic sulfur XANES resonances, ca. 2473 and 2483
eV, to map the distribution of reduced and oxidized sulfur species,
respectively.
The SXM images reveal high concentrations of organosulfur species in
lignin-rich parts of the wood structure, especially in the middle lamella
holding the cells together (Figure 3 (lower), at 2473 eV). Freshly
salvaged oak wood from the Mary Rose wreck site displayed reduced
sulfur (thiols) in a distinct double layer in the walls of a vessel, which is a
lignin reinforced channel for water transport, although how the lignin is
distributed is not known. The high concentration of organosulfur in lignin-rich
parts indicates direct reaction of the hydrogen sulfide (or HS-
ions) with active sites in lignin. This resembles the formation of organosulfur
species in humic matter, which may partly be composed of lignin. Cross-linking
of thiols via S-S bonds has been proposed to build macromolecular structures,
which may have geochemical importance for the stabilization of organic matter
in anoxic marine sediments and subsequently for the presence of sulfur in
fossil fuels (Vairavamurthy et al., 1997).
Focused micro-XANES spectra occasionally show iron sulfides in particles
(Fe1-xS, 0<x<0.1, with peak energy ~2471 eV), and deconvoluted SSRL
XANES spectra sometimes reveal pyrite FeS2. Microcrystalline iron
sulfides are known to be unstable towards oxidation in a humid environment, and
are probably the primary source of the acid that forms in the moist wood of the
shipwrecks. About 2 tons of sulfuric acid is estimated to have accumulated in
the Vasa's PEG impregnated hull after the spray treatment was stopped in
1979 (Sandström et al., 2003).
In the "Preserve the Vasa" project, tests are being carried out to
remove iron or in other ways slow down oxidation processes of the sulfur
compounds. Specially tailored derivatives of the well-known chelate EDTA, which
form water-soluble complexes with particularly strong bonds to iron(III), are
being tested for iron extraction. The spraying washes out the acid produced in
the hull timbers of the Mary Rose, and the rate of acid production is
carefully monitored to assess a suitable end point of the current spray
treatment. After the strongly acid-forming iron sulfides are exhausted,
antioxidants could be added to stabilize the lignin-bonded organosulfur
compounds and also to prevent PEG degradation. For the Vasa a new wet
spray treatment would be stressful for the degraded wood. Neutralizing the
accumulated acid with an ammonia gas treatment, followed by storage in stable
and low (~55%) relative humidity, may be sufficient to hold back the effects of
acid-forming oxidation processes. Monitoring the progress of the ongoing work
at synchrotron facilities, as well as analyses of new marine-archaeological
artefacts will have important influence on the direction of future conservation
efforts.
This research was in part carried out at the Stanford Synchrotron Radiation
Laboratory, a national user facility operated by Stanford University on behalf
of the U.S. Department of Energy, Office of Basic Energy Sciences. The SSRL
Structural Molecular Biology Program is supported by the Department of Energy,
Office of Biological and Environmental Research, and by the National Institutes
of Health, National Center for Research Resources, Biomedical Technology
Program.
Primary Citation:
M. Sandström, F. Jalilehvand, E. Damian, Y. Fors, U. Gelius, M. Jones, and M.
Salomé, "Sulfur accumulation in the timbers of King Henry VIII's warship Mary
Rose: A pathway in the sulfur cycle of conservation concern",
PNAS,
102 (40), 14165-14170 (2005).
References
compounds. Bacteria reduce sulfate ions in seawater to
dissolved hydrogen sulfide, H2S(aq), which penetrates and reacts to solid
sulfur compounds within the waterlogged wood. The accumulated amount of sulfur
depends on the state of wood degradation, the concentration of hydrogen sulfide
and of iron ions, as shown by comparisons of the sulfur compounds in wood from
shipwrecks preserved under different conditions (Sandström
et al., 2005). The
XANES spectra show that two classes of compounds are formed: 1.
organosulfur
compounds (e.g. thiols, disulfides, sulfoxides), 2. iron
sulfides (Fe1-xS and pyrite FeS2) and also elemental
sulfur S8. Iron sulfides may occur in large quantities in the wood
when reactive iron corrosion products have been present providing iron(II) ions
in excess. For the Mary Rose, the iron concentration in the wood
fluctuates considerably.
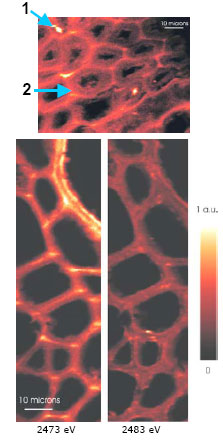
(lower) Freshly salvaged oak wood from the Mary Rose after 459 years on the
seafloor. The left SXM image at 2473 eV, displays two distinct layers of thiols
in the lignin-rich cell wall of a vessel (top right corner), where
liquids are transported in oak wood; the dark patches surrounded by cell walls
are the lumina. The 2483 eV SXM image to the right shows a few bright sulfate
particles and a diffuse sulfate background from seawater in the waterlogged
wood.
http://www-ssrl.slac.stanford.edu/research/highlights_archive/vasa.html.