
Scott Pegan1, Christine Arrabit2, Wei Zhou1, Witek Kwiatkowski1, Anthony Collins3, Paul Slesinger2 and Senyon Choe1
Structural Biology1 and Peptide Biology2 Laboratories, The Salk Institute, La Jolla, Ca 92037; Department of Pharmaceutical Sciences3, College of Pharmacy, Oregon State University, Corvallis, OR 97331
The family of inwardly-rectifying potassium (Kir) channels of eukaryotic cells
are unique because they conduct K+ ions better in the inward than
outward direction. In native tissues, the small outward K+ current
through Kir channels influences the resting membrane potential and membrane
excitability. The major structural mechanism underlying inward rectification
involves a physical occlusion of the pore by polyamines and Mg2+
from the cytoplasmic side of the channel1,2. In addition to the property of inward rectification, Kir
channels respond to a variety of intracellular messengers, including G proteins
(Kir3 channels), ATP (Kir6 channels) and pH (Kir1 channels)3. The aberrant activity of Kir channels has been linked to a
variety of endocrine, cardiac and neurological disorders. For instance, the
loss of Kir3 channels leads to hyperexcitability and seizures in the
brain4, cardiac abnormalities5 and hyperactivity and reduced anxiety. Mutations in Kir1
and Kir2.1 channels have been implicated for Bartter's syndrome6 and Andersen's syndrome7,
respectively. The high resolution structures of Kir 3.1 and Kir 2.1,
elucidated with data collected at SSRL 9-1 and ALS respectively, yielded
insight into the gating, inward rectification, and causes of Andersen's
Syndrome.
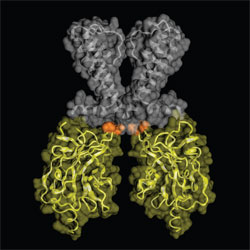
By comparing the Kir3.1 and Kir2.1 structures, a high degree of flexibility was
observed at the narrowest region of the channel's tetrameric pore, the G-loop
(Figure 1). The G-loop contains several small or hydrophobic residues and is
anchored by glycine. In the Kir3.1 structure solved at SSRL, the distance at
the narrowest point of the G-loop was 9.0 Å between the atomic centers of
diagonally-positioned subunits, which differed from the 5.7 Å distance observed
for equivalent positions of A306 in the Kir2.1 structure. Thus, the physical
opening formed by four opposing hydrophobic G-loops is too narrow to
accommodate a hydrated potassium ion to pass and leads us to conclude that the
Kir3.1 and Kir2.1 structures are of a closed state. Mutations, based on the
structure and studied by eletrophysiology, dramatically reduced the flexibility
of the G-loop. Bulky sidechains in the G-loop chains inhibited channel
current. These results reinforce the role of the G-loop to form the closed
state.
The elucidated structures not only showed insight into the gating of the Kir
family of channels but also lead to a better understanding of the inward
rectification properties of this family of channels. By studying the
electro-potential surfaces of the Kir3.1 and Kir2.1 structures, the Kir2.1
structure shows a remarkably high degree of electronegative surface potential
as compared to that of Kir3.1. Interestingly, a recent structure of
KirBac1.1's cytoplasmic pore exhibits less electronegative surface than Kir3.1.
Previously, the strong rectification of Kir2.1 has been attributed to two
principal electronegative regions; D172 in the M2 domain8 and E224/E299 in the cytoplasmic domains9,10. Using the structure of Kir2.1 as a
guide and electrophysiology experiments to confirm our findings, we identified
that D255 and D259 are linked to Kir2.1's strong rectification properties
unlike other members in the Kir family.
The Kir2.1 structure allowed the first structural understanding of Andersen's
Syndrome. Out of the eighteen positions in the Kir2.1, ten were visualized
with eight located on the top surface of the cytoplasmic structure (R189, T192,
R218, G300, V302, E303, R312, D314-315), which may
be near the punitive PIP2
(phosphatidylinositol-4,5-bisphosphate)-binding site, and the other two buried
in the protein interface (G215D, N216H). Some of these residues are
interestingly close to the G-loop region and generally result in a loss of
function via dominant negative interactions and heteromeric assembly
7. For all
but one mutation, G300V, the resulting mutant protein was aggregated, pointing
to folding and tetramerization defects as the main reason for the disease. To
validate the point, one of the mutations known to disrupt a charged pair
interaction, R218Q, was rescued from the folding defect by a compensating
mutation to R/K at T309 as predicted by the Kir2.1 structure.
The elucidated structures of the Kir2.1 and Kir3.1 cytoplasmic domains have
provided us with a broader understanding of how this channel gates and
rectifies itself. Furthermore, the electrophysiology experiment of the
Andersen's Syndrome mutates coupled with the structural information has allowed
for the first time to provide an explanation of how these mutations could
interfere with the folding and gating of the Kir2.1 channel. Our better
understanding may lead to therapeutic treatments for the disease.
Primary Citation:
References:
Pegan, S., Arrabit, C., Zhou W., Kwiatkowski W., Collins A., Slesinger
PA., Choe, S. (2005) Cytoplasmic domain structures of Kir2.1 and Kir3.1 shows
sites for modulating gating and rectification. Nat Neurosci. 8:
279-287
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |