

The water molecule, H2O, has deceptively simple structure, but
contains all the prerequisites for building complexity. The oxygen atom has a
greater affinity for electrons and pulls them away from the hydrogens making
them slightly positive. On the back side of molecule oxygen has a lone pair -
electrons that do not assist in binding the hydrogens in the molecule, but to
which the hydrogens of another water molecule can be attracted to form a
so-called hydrogen bond (H-bond). Hydrogen bond is much weaker than the bonding
inside water molecule, but it is still strong enough with the possibility to
make from one up to four H-bonds per water molecule. The network connected by
H-bonds between water molecules makes liquid water so special compared to other
normal liquids with about 66 anomalies, e.g. density maximum at 4 °C and large
heat capacity. The anomalies of water become extreme in the supercooled region
(below freezing point), whilst they are also present at ambient conditions
where most of waters' physical, chemical and biological processes of importance
occur. Water at ambient conditions has traditionally been considered as a
homogeneous distribution of near- tetrahedral H-bonded structures with thermal
fluctuations increasing with temperature. This picture has been challenged by
recent studies based on x-ray Raman (XRS), x-ray absorption spectroscopy (XAS)
and x-ray emission spectroscopy (XES), suggesting two distinct local structures
with tetrahedral as a minority and highly H-bond distorted asymmetrical as the
majority. In particular, the proposed predominant asymmetrical structure has
caused intense debate in the last years.
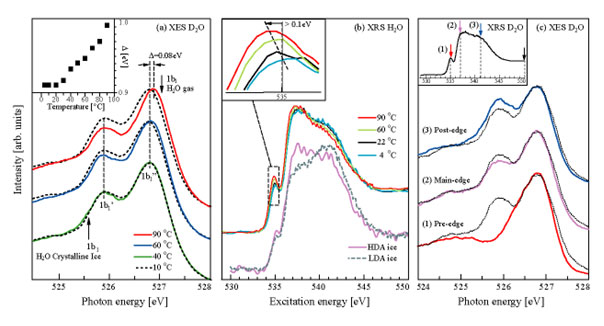
Figure 1a shows the temperature dependence in the lone pair 1b1
region of the XES spectra for D2O. All spectra show a split of the
lone pair into two peaks, denoted 1b1' and 1b1", where
the former is close in energy position to the 1b1 in crystalline ice
and the latter to 1b1 in water vapor. The two peaks can thereby be
related to tetrahedral (1b1') and H-bond distorted
(1b1") local structures with further support for this assignment
given below. Figure 1b top part shows the temperature-dependent XRS spectra
with energy resolution of 0.5 eV, which are denoted as pre-edge (535 eV),
main-edge (537-538 eV) and post-edge (540-541 eV). Tuning the energy to the
specific resonant features (pre-, main- and post-edge) in the absorption
spectrum makes a connection between XRS and XES by selecting the corresponding
structural species for XES. This is shown in Fig. 1c where resonant XES spectra
are compared with non-resonant (550 eV) XES. We find that pre-edge excitation
essentially eliminates the 1b1' (tetrahedral) peak (red),
excitation on the main edge gives a slight enhancement of the 1b1"
(distorted) (green), while excitation on the post-edge instead enhances the
1b1' (tetrahedral) peak compared to the 1b1" (blue).
Since the absorption post-edge feature in ice is much stronger than in the
liquid, the resonant XES (blue) is consistent with that the 1b1'
peak is related to tetrahedral-like species. The pre-edge peak in XRS has, on
the other hand, been assigned to distorted H-bonding configuration. This
assignment is consistent with the observed absence of the 1b1'
(tetrahedral) and the strong enhancement of the 1b1" (distorted)
peak when resonantly exciting on the pre-edge feature (red).
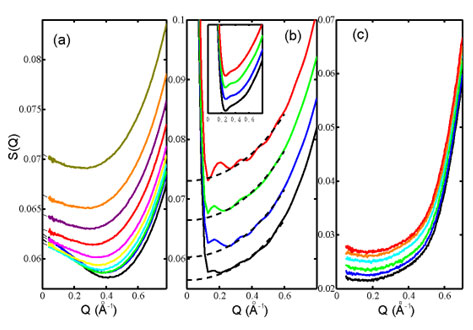
Small angle x-ray scattering (SAXS) give further sight into the length-scale on
which the two distinct local structures are fluctuated. Figure 2a shows the
normalized structure factor, S(Q), derived from the SAXS intensity in
ambient water at temperatures from 7 to 74 °C. All scattering curves show
an enhancement approaching Q = 0 after experiencing a minimum around
0.4-0.5 Å-1, which to first approximation directly indicates
the presence of density heterogeneities. In particular, the enhancement becomes
smaller with increasing temperature in strong contrast to expectation from
simple thermal density fluctuations. In order to address if the enhancement at
low Q can be related to and reproduced by thermal fluctuations in common
water models, we have performed molecular dynamics (MD) simulations as shown in
Figure 2b. The most important scattering enhancement observed at small
Q in the
experiment is completely missing from the SPC/E MD data even down to
Q = 0.13 Å-1. For comparison, Fig. 2c shows
S(Q) of CCl4 measured at temperatures from 6 to
30 °C and regarded as representing a "normal" liquid. It is clear that
SAXS of CCl4 shows no temperature-dependent variation at low
Q, which is
observed, in contrast, in the ambient water.
We analyze the SAXS data within the framework of Ornstein-Zernicke (OZ) theory
assuming the density fluctuations to result from the presence of either a
spinodal or critical point. The resulting OZ correlation length is around 3.1
Å, slowly decreasing with increasing temperature. The zero-angle
anomalous structure factor, on the other hand, is found to decrease more
dramatically with temperature. We further analyze the density fluctuations in
the SAXS data in terms of the picture indicated by XES and XRS, namely that, on
the time-scale of x-ray scattering, the liquid can be viewed as tetrahedral
patches surrounded by thermally excited H-bond distorted structures. Although
we lack information on the time-scale of the fluctuations, the attosecond
interaction time of the x-ray scattering process, compared to picoseconds for
H-bond dynamics, allows considering the SAXS data as an instantaneous snapshot
of the structure. Consequently, we infer a physical picture of the derived OZ
correlation length ξ in terms of the radius of gyration,
Rg. The relationship
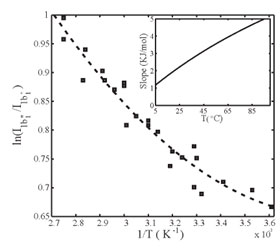
In Fig. 3, we fitted the XES spectra at each temperature into two spectral
components to obtain the intensity ratio I1b1"/I1b1'
between distorted and tetrahedral structures. We note that the data deviate
from a straight line, i.e. an Arrhenius behavior, indicating that energy and
entropy differences vary with temperature between the two local structures.
Since the tetrahedral structure in both XES and XRS shows little spectral
change with temperature we can assume that energy and entropy variation are
mainly attributable to changes of the distorted structure. The shifting of
1b1"
position in XES (Fig. 1a) and the pre-edge in XRS (Fig. 1b) with temperature
indicate an increase in entropy of the distorted component as it becomes
thermally excited. All of these observations are consistent with that the
tetrahedral structure is of lower energy - lower entropy and the distorted
structure of higher energy - higher entropy.
In summary, we use SAXS to demonstrate the presence of density fluctuations in
ambient water on a physical length-scale of around 1 nm. The length-scale is
retained while the magnitude of fluctuations is enhanced in ambient water with
decreasing temperature. In contrast, the magnitude of fluctuations in a normal
liquid of CCl4 exhibits little temperature-dependence. Based on XES
and XRS data we propose that the density difference contrast in SAXS is due to
fluctuations between tetrahedral-like and H-bond distorted structures related
respectively to low and high density water. We combine our experimental
observations to propose a model of water as a temperature-dependent,
fluctuating equilibrium between the two types of local structures driven by
incommensurate requirements for minimizing enthalpy (strong near-tetrahedral
hydrogen-bonds) and maximizing entropy (non-directional H-bonds and disorder).
The present results provide experimental evidence that the extreme differences
anticipated in the H-bonding environments in the deeply supercooled regime
surprisingly remain in bulk water even at conditions ranging from ambient up to
close to the boiling point.
Primary Citation
C. Huang, K. T. Wikfeldt, T. Tokushima, D. Nordlund, Y. Harada, U. Bergmann, M.
Niebuhr, T. M. Weiss, Y. Horikawa, M. Leetmaa, M. P. Ljungberg, O. Takahashi,
A. Lenz, L. Ojamae, A. P. Lyubartsev, S. Shin, L. G. M. Pettersson and A.
Nilsson, "The Inhomogeneous Structure of Water at Ambient Conditions",
Proc. Natl. Acad. Sci. USA 106, 15241 (2009) doi: 10.1073/pnas.0904743106
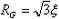 gives
RG decreasing from 5.6 at 7 °C to 5.1 Å at 74
°C. In order to get a sense of the physical dimensions of the tetrahedral
patches we assume a spherical shape giving a diameter, D, of 14.5-13.2
Å.
gives
RG decreasing from 5.6 at 7 °C to 5.1 Å at 74
°C. In order to get a sense of the physical dimensions of the tetrahedral
patches we assume a spherical shape giving a diameter, D, of 14.5-13.2
Å.
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences.