 Nanometer-size metal particles are of fundamental interest for their chemical
and quantum electronic properties, and of practical interest for many potential
applications [1, 2]. Historically gold
nanoparticles are the best studied, dating back to ancient Rome where colloidal
gold was thought to have medicinal properties due to its blood red color. Gold
has proven to have applications in medicine with some modern arthritis drugs
using gold compounds. With all this interest in gold particles the structure
of a thiol-protected gold nanoparticle had yet to be unambiguously determined.
Electron microscopy
Nanometer-size metal particles are of fundamental interest for their chemical
and quantum electronic properties, and of practical interest for many potential
applications [1, 2]. Historically gold
nanoparticles are the best studied, dating back to ancient Rome where colloidal
gold was thought to have medicinal properties due to its blood red color. Gold
has proven to have applications in medicine with some modern arthritis drugs
using gold compounds. With all this interest in gold particles the structure
of a thiol-protected gold nanoparticle had yet to be unambiguously determined.
Electron microscopy
| |
| 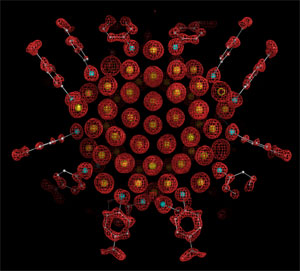 |
|
Fig. 1. X-ray crystal structure determination of Au102
(p-MBA)44
nanoparticle. Electron density map (red mesh) and atomic structure (gold atoms
depicted as yellow spheres, p-MBA shown as framework and small spheres sulfur
in cyan, carbon in gray and oxygen in red). [full-sized view] |
| [3,4],
powder x-ray diffraction [5] and theoretical studies
[6] had led to the
idea that gold nanoparticles would adopt closed geometric shells with
crystalline packing. This would lead to defined core sizes such that the gold
clusters would have a discrete number of atoms representing closed geometric
shells [7]. For example icosahedrally packed gold clusters were predicated to
have "magic number" sizes corresponding to 55, 147, 309. An alternative to the
closed geometric model was the jellium model, which predicted closed electronic
shells instead of geometric shells (reviewed in Rev. Mod. Phys 65, 611-676).
Testing of these theories requires the unambiguous structural determination of
a series of gold nanoparticles.
Structural determination of thiol protected gold nanoparticles has been
complicated by the problem that particles are typically heterogenous as
synthesized. Through systematic variation of solution conditions for gold
nanoparticle synthesis, the Kornberg lab obtained particles sufficiently
uniform in size for the growth of large single crystals opened the way to X-ray
structure determination. SSRL beamlines 11-1 and 11-3 were utilized to perform
X-ray analysis of these crystals resulting in the first unambigous
determination of a thiol protected gold nanoparticle. The resulting electron
density map revealed a particle of 102 gold atoms and 44 p-mercaptobenzoic
acids (p-MBAs) (Figure 1). The structure was refined at a resolution of 1.15
Å to Rwork and Rfree of 8.8% and 9.5%.
| 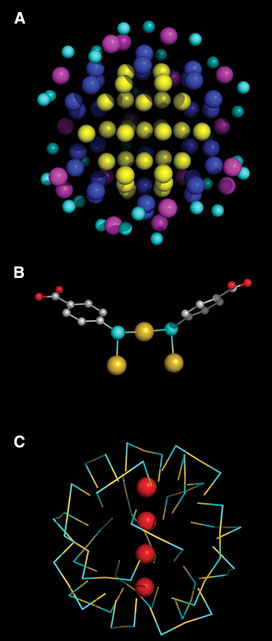 |
|
Fig. 2. Sulfur-gold interactions in the surface of the nanoparticle.
(A) Successive shells of gold atoms, interacting with zero (yellow), one
(blue), or two (magenta) sulfur atoms. Sulfur atoms are cyan. (B) Example of
two p-MBAs interacting with three gold atoms in a bridge conformation, here
termed a "staple motif." Gold atoms are yellow, sulfur cyan, oxygen red.
(C)
Distribution of staple motifs in the surface of the nanoparticle. Staple
motifs are depicted symbolically, with gold in yellow and sulfur in cyan.
Only the gold atoms on the axis of the MD are shown, in red. |
|
The Au102(p-MBA)44 structure revealed a metallic gold
nucleus of 79 atoms packed with decahedral symmetry protected by a
Au23(p-MBA)44 layer of gold-thiol oligomers. Gold atoms
up to 5.5 Å from the center of the particle do not contact sulfur, those in a
shell of radius 6.0 to 6.3 Å bind one sulfur, and those in a shell of radius
7.5 to 8.0 Å bind two sulfurs (Fig. 2A). All sulfur atoms lie in a shell of
radius 8.3¬ ± 0.4 Å and bind in a bridge conformation [8] to
two gold atoms, at least one of which binds two sulfurs, forming a "staple"
motif (Fig. 2B, C). The gold-sulfur distance ranges from 2.2 to 2.6 Å.
Gold-sulfur-gold angles are 80 to 115° and sulfur-gold-sulfur angles are 155 to
175°. If the surface is taken as all gold atoms interacting with sulfur, then
the coverage by p-MBA (thiol:gold ratio) is 70%, much higher than the values of
31% and 33% for benzenethiol [9] and alkanethiols [10] on Au(111) surfaces, reflecting the curvature of the
nanoparticle surface.
The very existence of a discrete Au102(p-MBA)44
nanoparticle is a notable finding from this work. Discrete sizes have been
explained in the past by geometrical or electronic shell closing. The
arrangement of gold atoms, with polar caps and an equatorial band, argues
against geometrical shell closing. If, however, each gold atom
(5d10 6s1)
contributes one valence electron, and 44 are engaged in bonding to sulfur, then
58 electrons remain, corresponding to a well-known filled shell. Indeed, a
naked cluster in the gas phase containing 58 gold atoms shows exceptional
stability [11, 12].
There are a number of connections of the Au102 nanoparticle
structure with previous work. First, structures of small gold, silver, and
platinum clusters, and of large platinum-palladium clusters, include five-fold
symmetry elements and also, in one case, thiols bridging between pairs of gold
atoms [13-16]. Second, electron
microscopy, X-ray powder diffraction and theoretical studies of large gold
clusters have given results consistent with a decahedron [3-
13]. Third,
theoretical studies have raised the possibility of distinct gold-sulfur units
capping a central gold core [17]. Fourth, the Face centered cubic (fcc)
packing in the core, with a gold-gold distance of 2.8-3.1 Å, corresponds with
the fcc packing in bulk metallic gold, with a gold-gold distance of 2.9 Å.
Fifth, the staple motif, containing alternating gold and sulfur atoms,
resembles the gold-thiol polymers believed to represent intermediates in the
process of nanoparticle formation [18]. Finally, circular dichroism
measurements on gold nanoparticle preparations have shown chiro-optical
activity [19].
We have screened fifteen crystals derived from multiple gold nanoparticle
preparations and obtained the same gold-102 structure. Other nanoparticle
preparations, however, which have also given rise to large single crystals,
will doubtless reveal other core structures, from which rules or principles of
core assembly may ultimately be derived. It remains to investigate the
chemical and physical properties of the Au102 nanoparticle, as well
as to explore the theoretical basis of the gold packing and gold-thiol
interactions we have observed.
Primary Citation
Jadzinsky, P.D., G. Calero, C. J. Ackerson, D. A. Bushnell and R. D. Kornberg,
Structure of a Thiol Monolayer-Protected Gold Nanoparticle at 1.1 Å Resolution.
Science, 2007. 318, p. 430-433.
References:
-
Brust, M. and C.J. Kiely, Monolayer protected clusters of gold and silver.
Colloids and Colloid Assemblies, 2004: p. 96-119.
-
Daniel, M.-C. and D. Astruc, Gold nanoparticles: assembly,
supramolecular chemistry, quantum-size-related properties, and applications
toward biology, catalysis, and nanotechnology. Chemical reviews, 2004.
104(1):
p. 293-346.
-
Yacaman, M.J., et al., Structure shape and stability of nanometric
sized particles. Journal of Vacuum Science & Technology B-an International
Journal Devoted to Microelectronics and Nanometer Structures-Processing
Measurement and Phenomena, 2001. 19(4): p. 1091-1103.
-
Ascencio, J.A., et al., Structure determination of small particles by
HREM imaging: theory and experiment. Surface Science, 1998. 396(1-3): p.
349-368.
-
Cleveland, C.L., et al., Structural Evolution of Smaller Gold
Nanocrystals: The Truncated Decahedral Motif. Physical Review Letters, 1997.
79(10): p. 1873-1876.
-
Aiken, J.D. and R.G. Finke, A review of modern transition-metal
nanoclusters: their synthesis, characterization, and applications in
catalysis.
Journal of Molecular Catalysis a-Chemical, 1999. 145(1-2): p. 1-44.
-
Martin, T.P., Shells of atoms. Physics Reports-Review Section of
Physics Letters, 1996. 273(4): p. 199-241.
-
Bau, R., Crystal Structure of the Antiarthritic Drug Gold Thiomalate
(Myochrysine): A Double-Helical Geometry in the Solid State. Journal of the
American Chemical Society, 1998. 120(36): p. 9380-9381.
-
Wan, L.J., et al., Molecular Orientation and Ordered Structure of
Benzenethiol Adsorbed on Gold(111). J. Phys. Chem. B, 2000. 104(15): p.
3563-3569.
-
Vericat, C., M.E. Vela, and R.C. Salvarezza, Self-assembled monolayers
of alkanethiols on Au(111): surface structures, defects and dynamics. Physical
Chemistry Chemical Physics, 2005. 7(18): p. 3258-3268.
-
de Heer, W.A., The physics of simple metal clusters: experimental
aspects and simple models. Rev. Mod. Phys., 1993. 65: p. 611-676.
-
Martin, T.P., et al., Shell structure of Clusters. J. Phys. Chem.,
1991. 95: p. 6421-6429.
-
Mednikov, E.G., M.C. Jewell, and L.F. Dahl, Nanosized
(m12-Pt)Pd164-xPtx
(CO)72(PPh3)20 (x approximately 7)
Containing Pt-Centered Four-Shell 165-Atom Pd-Pt Core with Unprecedented
Intershell Bridging Carbonyl Ligands: Comparative Analysis of Icosahedral
Shell-Growth Patterns with Geometrically Related
Pd145(CO)x(PEt3)30
(x approximately 60) Containing Capped Three-Shell Pd145 Core. J. Am.
Chem. Soc., 2007. 129: p. 11619-11630.
-
Shichibu, Y., Biicosahedral Gold Clusters
[Au25(PPh3)10(SCnH2n+1)5Cl2]2+ (n = 2-18): A Stepping Stone to
Cluster-Assembled Materials. Journal of Physical Chemistry C 2007.
111(22): p. 7845-7847.
-
Teo, B.K. and H. Zhang, Synthesis and Structure of a Neutral
Trimetallic Biicosahedral Cluster,
(Ph3P)10Au11Ag12Pt2Cl7.
A Comparative Study
of Molecular and Crystal Structures of Vertex-Sharing Biicosahedral Mixed-Metal
Nanoclusters. Journal of Cluster Science 2001. 12(1): p. 349-383.
-
Teo, B.K. and e. al., Rotamerism and roulettamerism of vertex-sharing
biicosahedral supraclusters: Synthesis and structure of
[(Ph3P)10Au13Ag12Cl8](SbF6). Journal of Cluster Science 1993. 4(4):
p. 471-476.
-
Hakkinen, H., M. Walter, and H. Gronbeck, Divide and protect: Capping
gold nanoclusters with molecular gold-thiolate rings. Journal of Physical
Chemistry B, 2006. 110(20): p. 9927-9931.
-
Templeton, A.C., W.P. Wuelfing, and R.W. Murray, Monolayer-Protected
Cluster Molecules. Accounts of Chemical Research, 2000. 33(1): p. 27-36.
-
Schaaff, T.G. and R.L. Whetten, Giant Gold-Glutathione Cluster
Compounds: Intense Optical Activity in Metal-Based Transitions. Journal of
Physical Chemistry B, 2000. 104(12): p. 2630-2641.
|
| PDF
Version | | Lay Summary | |
Highlights Archive
|
| SSRL is supported
by the Department of Energy, Office of Basic Energy Sciences. The SSRL
Structural Molecular Biology Program is supported by the Department of Energy,
Office of Biological and Environmental Research, and by the National Institutes
of Health, National Center for Research Resources, Biomedical Technology
Program, and the National Institute of General Medical Sciences. |
|

 Nanometer-size metal particles are of fundamental interest for their chemical
and quantum electronic properties, and of practical interest for many potential
applications [1, 2]. Historically gold
nanoparticles are the best studied, dating back to ancient Rome where colloidal
gold was thought to have medicinal properties due to its blood red color. Gold
has proven to have applications in medicine with some modern arthritis drugs
using gold compounds. With all this interest in gold particles the structure
of a thiol-protected gold nanoparticle had yet to be unambiguously determined.
Electron microscopy
Nanometer-size metal particles are of fundamental interest for their chemical
and quantum electronic properties, and of practical interest for many potential
applications [1, 2]. Historically gold
nanoparticles are the best studied, dating back to ancient Rome where colloidal
gold was thought to have medicinal properties due to its blood red color. Gold
has proven to have applications in medicine with some modern arthritis drugs
using gold compounds. With all this interest in gold particles the structure
of a thiol-protected gold nanoparticle had yet to be unambiguously determined.
Electron microscopy

