O. Pornillos, Y-J. Chen, A. P. Chen and G. Chang
Department of Molecular Biology, The Scripps Research Institute, La Jolla,
CA 92037
|
Using the x-ray diffraction data collected on BL11-1 at SSRL, ALS, and APS,
Geoffrey Chang's group at The Scripps Research Institute has solved the crystal
structure of EmrE multidrug transporter in complex with a substrate,
tetraphenylphosphonium (TPP). The data for the selenomethionine labeled protein
was collected at SSRL. The structure was determined to 3.7 Å resolution by
anomalous dispersion methods, using the arsonium analog of TPP and
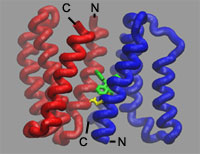
selenomethionine-susbstituted protein. This membrane protein is a homodimer
made of two chemically but not structurally identical polypeptides that align
themselves in an inverted, antiparallel fashion. Although the subunits have the
same amino acid sequence, they adopt different conformations, making the
protein asymmetric. Each subunit has four helices. The arrangement of the first
three helices is nearly identical in each subunit; the fourth helix, however,
is packed differently. The difference between the fourth helices provides the
structural basis for the asymmetry and explains how the transporter could have
a function that's unidirectional. Two EmrE polypeptides from a homodimeric
transporter bind the substrate at the dimerization interface. The structure
also shows the location of two glutamates that have previously been shown
through biochemical experiments to be essential for drug efflux.
This work was supported by grants from the NIH (GM67644 and GM073197) and NASA
(NAG8-1834).
Primary Citation
Pornillos, O., Chen, Y-J., Chen, A. P., and Chang, G. (2005) X-ray Structure of
the EmrE Multidrug Transporter in Complex with a Substrate.
Science 310,
1950-1953.
References
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |