

 | |
Figure 1: (a) Warrior # T18G21-08, a kneeling archer. The pigment samples in this study have been taken from this terracotta warrior. (b) Close-up picture of the purple paint on the terracotta warrior. (c) Images of the purple paint samples used in this study. | |
In March 1974 during the sinking of wells for farmland irrigation near Xi'an,
China, nine farmers made one of the world's most remarkable archaeological
finds: the discovery of an army consisting of more than 8000 life-size terra
cotta figures of warriors and horses of the First Emperor of Qin. One of the
most intriguing puzzles is the purple synthetic pigments ("Chinese Purple" or
"Han Purple" [1]) found on the terra cotta soldiers (figure
1). Until the 19th century, most pigments were based on naturally occurring
colored minerals and dyes, with three significant exceptions: Egyptian Blue
(CaCuSi2O10), Chinese Blue
(BaCuSi2O10)/ Purple (BaCuSi2O6)
and Maya Blue. The former two are alkaline-earth copper silicates, and because
of this similarity it has been proposed that the Chinese pigments were derived
from Egyptian Blue [2].
This supposition, however, leaves many open questions. First, it is unlikely
that the Chinese chemists could have acquired the technology (not just the
pigment) from Egypt well before the official "silk road" (125 BC). Some
earliest Chinese Purple samples date back to the "Warring States" period
(479-221 BC). Considering the time needed to switch a calcium-based technology
to a barium-based one, this technology transfer, if there was one, must have
happened well before the "Warring States" period. But even if there existed a
connection between China and Egypt, it does not explain why the Chinese decided
to substitute Ba for Ca and face the challenges associated with the consequent
elevation of the synthesis temperature. An additional problem with this theory
is that, to our knowledge, no Ca-bearing Egyptian Blue has been found in China.
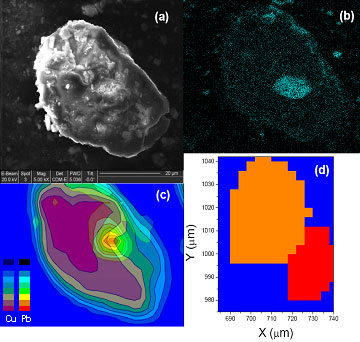
Figure 2: (a) SEM image of the pigment clump taken at 20KeV.
(b) Lead (Pb La) concentration EDX map of the
same clump is taken at 15keV. (c) We show an overlapping mXRF (1.5mm spot size)
concentration map of Pb (light green, yellow, and orange regions) and Cu (dark
green and purple regions) taken at 14KeV. mXRF
provides more bulk sensitive information than the EDX microanalysis.
(d) The detailed crystallographic map derived from the mXRD (1.2mm spot size) scan. It
shows that there are two pigment grains (in orange and red) in the clump which
have slightly different crystallographic orientations. The Pb compounds are
found either in between the two grains or on the boundary of the grains.
In summary, we argue that Chinese Purple was invented by Taoist alchemists as a
by-product of the technology originally developed for synthesizing
barium-containing glasses, which, in turn, were invented for the purpose of
imitating jade. The barium compounds were added to increase the refractive
index of the glass, thus giving the glass a similar appearance as jade. The
development of this process also benefited from two well-developed technologies
in ancient China: the earlier Bronze making (adding lead compounds to reduce
the melting temperature) and pottery making (advanced pottery kilns)
technologies.
It is remarkable that three ancient civilizations, Egypt, China and Maya,
invented their own blue pigments independently. The evolution of Chinese
purple and blue pigments is also a good example of how cultural changes in the
society affected the science and technology development in ancient China.
Primary Citation
References
In our study, we analyzed clumps of pigment (figure 2) from the Qin warriors
and discovered that in spite of the structural similarity to Egyptian Blue, the
micro-structural morphology of Chinese Purple is very different. Based on the
morphology and the phase distribution in the clumps of the pigment, it appears
that the process by which Chinese Purple was synthesized is very similar to
that of barium-containing glasses [3] Combining these results
with other archaeological evidence, we propose that the synthesis technology
for the Chinese pigments was in fact a by-product of high-refractive index
glasses (artificial jades) produced by Taoist monks. Furthermore, the
disappearance of these pigments from Chinese art and monuments concurrently
with the decline of Taoism substantiates the link between the two.
Z. Liu, A. Mehta, N. Tamura, D. Pickard, B. Rong, T. Zhou, P. Pianetta,
"Influence of Taoism on the invention of the purple pigment used on the Qin
terracotta warriors", J. Archaeol. Sci. (2007), doi:10.1016/j.jas.2007.01.005
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |