

 | |
Figure 1 Structure of the HcB-Syt-II complex. a, sA-weighted FO - FC electron density map (contoured at 1.5 s) around Syt-II, overlaid with the final refined model (Syt-II: red and green; HcB: grey). Please note that this map is model-bias free since it is calculated from the phases of the atomic model prior to the inclusion of the Syt-II peptide (using a lower resolution diffraction data set to 2.6 Å). b, Structure of the complex between HcB (salmon and gold) and Syt-II (red). | |
Botulinum neurotoxins (BoNTs) are produced by Clostridium botulinum and
cause the neuroparalytic syndrome of botulism. With a lethal dose of 1 ng/kg,
they pose a biological hazard to humans and a serious potential bio-weapon
threat (1). On the other hand, BoNTs have become a powerful
therapeutic tool in the treatment of a variety of neurological, ophthalmic, and
other disorders manifested by abnormal, excessive, or inappropriate muscle
contractions. Experimental studies are also underway that explore the use of
BoNTs in the management of chronic pain, such as headache and migraine. BoNTs
bind with high specificity at neuromuscular junctions and they impair
exocytosis of synaptic vesicles containing acetylcholine through specific
proteolysis of SNAREs which constitute part of the synaptic vesicle fusion
machinery (2,3). The molecular details of the
toxin-cell recognition have been elusive.
Figure 2
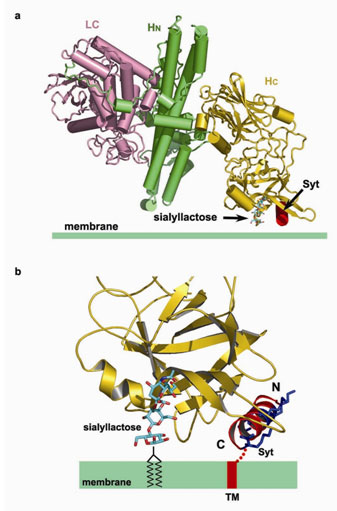
The simultaneous binding with membrane-anchored Syt-II and ganglioside imposes
geometric restrictions on how BoNT/B binds to the membrane surface. a,
Proposed binding mode of BoNT/B on the membrane surface. The structure of a
sialyllactose bound BoNT/B (PDB code: 1F31) was superimposed with the complex
of HcB-Syt-II using the coordinates of the Hc fragment for the alignment. The
light chain (LC), the N-terminal part of the heavy chain (HN), and
the C-terminal domain of the heavy chain (HC) are shown in pink,
green, and gold, respectively. b, A close-up view of the proposed
interface between BoNT/B and membrane. Four lysine residues that are conserved
among Syt-I and Syt-II are colored blue.
Primary Citation
References
Using the X-ray diffraction data collected on SSRL beam line 9-1 and ALS beam
line 8.2.2, Axel Brunger's group at Stanford University has determined the
first crystal structure of a BoNT in complex with its protein receptor: the
receptor binding domain (HcB) of botulinum neurotoxin serotype B (BoNT/B) bound
to the luminal domain of synaptotagmin II (Syt-II), at 2.15 Å resolution
(Figure 1). Upon binding a helix is induced in the luminal domain which binds
to a saddle-shaped crevice on a distal tip of BoNT/B. This crevice is adjacent
to the non-overlapping ganglioside binding site of BoNT/B (4,5) (Figure 2). Synaptotagmin II interacts with
BoNT/B with nanomolar affinity, at both neutral and acidic endosomal pH.
Biochemical and neuronal ex vivo studies of structure-based mutations
indicate high specificity and affinity of the interaction, and high selectivity
of BoNT/B towards the isoform II of synaptotagmin compared to isoform I.
Synergistic binding of both synaptotagmin and ganglioside imposes geometric
restrictions on the initiation of BoNT/B translocation upon endocytosis (Figure
2). These results could provide the basis for the rational development of
preventive vaccines or inhibitors against these neurotoxins. Furthermore,
identification of both receptor sites provides a new approach to retarget BoNTs
to different cell types by site directed mutagenesis. Such modified BoNTs could
also be used as drug delivery systems.
Jin, R., Rummel, A., Binz, T., and Brunger, A. T. (2006) Botulinum neurotoxin B
recognizes its protein receptor with high affinity and specificity.
Nature, 444, 1092-1095.
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |