
For many people, arsenic is synonymous with poison, so it is perhaps a surprise
that some plants, such as the fern Pteris vittata (Figure 1) seem to
quite deliberately accumulate large amounts of it. What is more, the plant
converts it to the most toxic inorganic form known. How does it do this?
First some background; while there is some evidence that arsenic is required
for health [1], this is debatable. On the other hand, the
poisonous nature of arsenic compounds was understood by the ancient Greeks and
Romans, and it has been used throughout history as a homicidal and suicidal
agent. It is found in two environmentally common oxy acids; arsenous acid
(H3AsO3), and arsenic acid
(H3AsO4), whose salts are known as arsenites and
arsenates, respectively. Of these, the trivalent arsenic species are the most
toxic. The infamous agent of murder is arsenic trioxide (white arsenic or
As2O3), which is simply the (reputedly tasteless)
anhydride of arsenous acid.
|
 |
|
Figure 1.
Pteris vittata sporophyte
|
The fern P. vittata takes up arsenate from soils [2],
transforms it to the more toxic arsenite [3], and stores it
within its tissues. Quite remarkable levels of arsenic can be accumulated - up
to 1 % dry weight. The plant, which presumably does this to prevent itself from
being eaten, is quite unaffected by its toxic cargo, and even seems to grow
slightly better in its presence. Pickering and co-workers have used X-ray
Absorption Spectroscopy Imaging, conducted on SSRL's BL9-3, to begin to unravel
how the fern carries out this fascinating process. Their technique
[4] combines X-ray absorption fluorescence microprobe maps
taken at carefully selected energies to yield beautiful images of the
distribution of the arsenic species in vivo.
Since ferns have two distinct generations in their life cycle - the familiar
leafy sporophyte (Figure 1), and the sexually active but rather tiny
gametophyte - Pickering et al. investigated both generations. They found that
arsenate is transported through the vascular tissue from the roots to the
fronds (leaves), and only there is it reduced to arsenite and apparently stored
in the vacuole. Arsenic-thiolate species observed surrounding the veins in the
leaves may be intermediates in this reduction. Arsenic was found to be excluded
from reproductive areas (spores and sporangia), and concentrated within nearby
sterile hairs or paraphyses. Being only one cell thick, the gametophytes are
ideal for studying the arsenic distribution within cells. Pickering et al.
showed that arsenite is compartmentalized within the large central cell vacuole
(Figure 2). In the gametophyte arsenic was found to be excluded from cell
walls, rhizoids and reproductive areas.
| 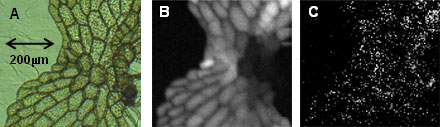 | | |
P. vittata gametophyte, optical micrograph (A) and XAS images of arsenite (B)
and arsenate (C), showing localization of arsenite in the large central cell
vacuole, and discrete speckles of arsenate are localized in unknown
sub-cellular compartments (possibly plant Golgi bodies).
| |
| | |
The study demonstrates the strength of the in situ capabilities of XAS
imaging by directly visualizing physiological processes in living plant
tissues. The study also provides insights which may prove useful for
phytoremediating arsenic-contaminated sites.
Primary Citation
Pickering, I.J., Gumaelius, L., Harris, H.H., Prince, R.C., Hirsch, G., Banks,
J.A., Salt, D.E., George, G.N. "Localizing the biochemical transformations of
arsenate in a hyperaccumulating fern". Environ. Sci. Technol.
,
2006, 40, 5010-5014.
References
-
Nielsen, F.H. Biol Trace Elem Res. 1990, 26-27, 599-611.
-
Ma L.Q.; Komar K.M.; Tu C.; Zhang W.; Cai Y.; Kennelley E.D. Nature
2001, 409, 579.
-
Webb, S.M.; Gaillard, J-F; Ma, L.Q.; Tu, C. Environ. Sci. Technol.,
2003, 37, 754-760.
-
Pickering, I.J., Prince, R.C., Salt, D.E., George, G.N. Proc. Natl.
Acad. Sci. U.S.A. 2000, 97, 10717-10722.
|
| PDF Version | | Lay Summary
|
| Highlights
Archive |
|
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences.
The SSRL Structural Molecular Biology Program is supported by the Department of
Energy, Office of Biological and Environmental Research, and by the National
Institutes of Health, National Center for Research Resources, Biomedical
Technology Program, and the National Institute of General Medical Sciences.
|
|
| Last
Updated: |
25 SEP 2006 |
| Content
Owner: |
Ingrid Pickering and Graham George, University of Saskatchewan
|
| Page
Editor: |
Lisa
Dunn |
|



