 Christopher S.
Kim,1 James J. Rytuba,2 Gordon E. Brown, Jr.3
Christopher S.
Kim,1 James J. Rytuba,2 Gordon E. Brown, Jr.3
1Department of Physical Sciences, Chapman University, Orange, CA
92866
2U.S. Geological Survey, Menlo Park, CA 94025
3Department of Geological and Environmental Sciences, Stanford
University, Stanford, CA 94305
Introduction
| |
|
 |
|
|
Figure 1.
Dr. Christopher Kim collects a mine waste sample from the Oat Hill mercury mine
in Northern California. The majority of mercury mine wastes at these sites are
present as loose, unconsolidated piles, facilitating the transport of
mercury-bearing material downstream into local watersheds.
|
|
Mercury (Hg) is a naturally occurring element that poses considerable health
risks to humans, primarily through the consumption of fish which have
accumulated harmful levels of mercury in their tissue. This bioaccumulation of
mercury in fish is a result of elevated exposure from a number of sources
including industrial emissions, atmospheric deposition, and mercury-bearing
products such as thermometers, batteries, electrical switches, and fluorescent
light bulbs. In California, however, a significant amount of mercury enters
the environment through the dozens of mercury mines located throughout the
California Coastal Range (Figure 2), where thousands of tons of mercury were
recovered for use in gold recovery further east in the Sierra Nevada. The
transport of mercury from these remote poorly-monitored mine sites has resulted
in elevated mercury levels in more populated urban regions such as the San
Francisco Bay Area. Understanding the movement and geochemistry of mercury
from mines in California is therefore necessary in order to predict the
potential impacts and hazards associated with this form of mercury
contamination.
The speciation of mercury is a critical determinant of its mobility,
reactivity, and potential bioavailability in mercury- and gold-mine impacted
| |
|
 |
|
|
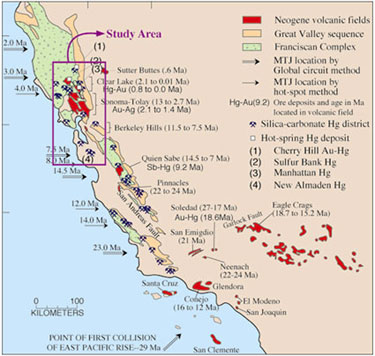
Figure 2.
Geologic map of mercury mining areas in the California Coast Range,
distinguishing between silica-carbonate deposits and hot-spring Hg deposits.
Samples were primarily collected from a variety of mine waste media at multiple
sites in the outlined region. From Rytuba (12).
(click on image for larger view of map)
|
|
regions. Mercury speciation in these complex natural systems is additionally
influenced by a number of physical, geological, and anthropogenic variables. In
order to investigate the degree to which several of these variables may affect
mercury speciation, extended x-ray absorption fine structure (EXAFS)
spectroscopy was conducted at SSRL beamlines 4-3 and 11-2 to determine the
mercury species and relative proportions of these species present in
mercury-bearing wastes from selected mine-impacted regions in California and
Nevada. This work represents the first in situ, non-destructive method by
which to identify mercury speciation in natural samples.
Mercury speciation protocol
The EXAFS-based speciation procedure for Hg is similar to that previously
developed for and applied to other metal(loid)s such as arsenic
(1,2), lead
(3-5), and
zinc (6,7). It involves the
comparison of an EXAFS spectrum collected from a Hg-bearing sample with a
spectral library of model compounds previously generated from an assortment of
Hg minerals and sorbed species (Figure 3). While each model compound spectrum
serves as the spectral fingerprint of a single Hg species, the EXAFS spectrum
| |
 |
|
|
|
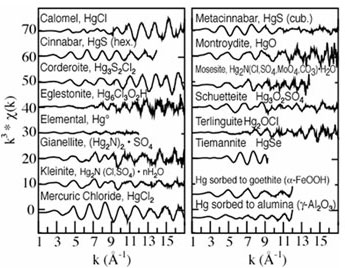
Figure 3.
Mercury model compound database of a host of Hg minerals and sorbed species.
Each Hg LIII-edge EXAFS spectrum is distinct from the others, reflecting the
unique structural environment around Hg from phase to phase.
(click on image for larger view)
| |
|
of a heterogeneous natural sample represents a composite of the EXAFS
contributions from all detectable Hg species present. Therefore, an EXAFS
spectrum collected from a natural sample containing multiple Hg species can be
decomposed using a linear least-squares fitting method into the sum of its
individual components through direct comparison with the model compound
spectra. Furthermore, determining the relative proportion of each model
compound's contribution to the least squares linear combination fit directly
yields the molar percentage of the respective Hg species identified in the
sample.
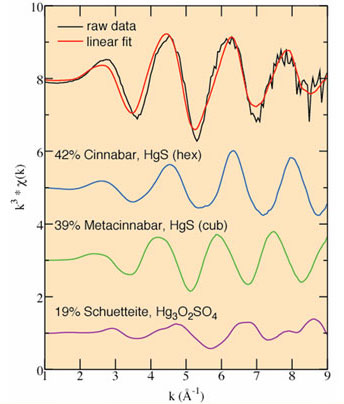
Figure 4 presents an example of such a linear fit, showing the fitted Hg
LIII-EXAFS spectrum of a Hg-bearing sample from the Bessels Mill in
Nevada and the related fit components. In this case, cinnabar (HgS, hex.),
metacinnabar (HgS, cub.), and schuetteite
(Hg3O2SO4) have been identified as
the significant components contributing to the final fit; when scaled to 100%,
they contribute in proportions of 42% and 39%, and 19%, respectively. This
identification of the relevant Hg species present and the proportions at which
they are present in the sample represents the formal, EXAFS-determined Hg
speciation of the sample. Application of the EXAFS technique to determine Hg
| |
|
 |
|
|
Figure 4.
Linear fitting results for a sample from the Bessels Mill, Carson River, NV,
showing the raw data (top, in black) best linear combination fit (top, in gray)
and the components (below) of the linear fit, scaled to the proportions to
which they contribute to the fit.
|
|
speciation in calibrated mixtures of model Hg compounds (8) and a separate
comparison of results from EXAFS spectroscopy and sequential chemical
extractions on a selected suite of Hg-bearing samples (9) further validated the
use of EXAFS spectroscopy for determining Hg speciation in complex samples and
better defined its limitations, where fit components should be considered
accurate to ±25% of their stated value and fit components comprising less than
10% of a fit should be viewed with caution.
Sampling
Samples were collected from a number of inactive Hg mine sites located along
the California Coast Range Hg mineral belt. Hg-contaminated samples from
former gold mine workings were also collected from the Carson River basin in
western Nevada. The sampled media included calcines (roasted ore), condenser
soot, waste rock (unroasted low-grade Hg-bearing material), distributed
sediments, Hg-bearing gold mine tailings, and an amorphous Fe-oxyhydroxide
precipitate forming downstream from an acid mine drainage seep at a Hg mine.
Most samples contained Hg concentrations of 100 mg/kg (ppm) or greater,
although a few samples with Hg concentrations below 100 ppm were also
successfully analyzed using EXAFS spectroscopy.
Effect of Geological Background on Hg Speciation
Since Hg ore deposits are known to form in a variety of geological settings, a
comparison of Hg speciation in mine wastes between different ore depositional
environments was expected to reveal fundamental differences in the types of Hg
species present. Speciation results determined by EXAFS spectroscopy of
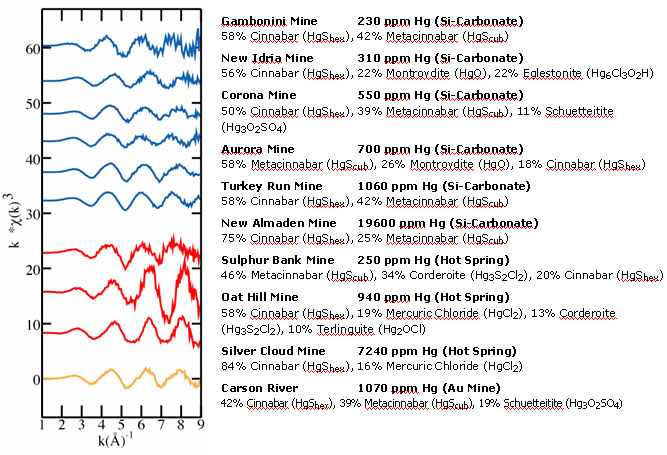
Hg-contaminated mine wastes from California and Nevada are shown in Figure 5.
Briefly, there are two primary types of Hg deposits in the California Coast
Range: (1) silica-carbonate Hg deposits, in which Hg is deposited in the highly
fractured zones of hydrothermally altered serpentinite bodies emplaced along
fault zones such as the San Andreas Fault, and (2) hot-spring Hg deposits,
where Hg is deposited in low-temperature, near-surface active hydrothermal
systems enriched in chloride and sulfate (10).
 |
|
Figure 5.
Summarized linear fitting results for a variety of Hg-bearing mine wastes, with
original EXAFS spectra shown.
|
The majority of Hg in all samples was found by EXAFS analysis to be present as
Hg-sulfides, either as cinnabar or metacinnabar; this finding is common to
nearly all global Hg deposits. Corderoite
(Hg3S2Cl2), a Hg-sulfide-chloride, was also
observed in two samples from hot-spring Hg deposits. In addition, minor
proportions of non-Hg-sulfide species, including as mercuric chloride
(HgCl2), eglestonite (Hg6Cl3O2H),
montroydite (HgO), schuetteite (Hg3O2SO4), and
terlinguite (Hg2OCl), were identified in several samples. The high
solubility of many of these species compared to the extremely insoluble
Hg-sulfides suggests that, although comprising a smaller percentage of the
total Hg in the sample, these species may be disproportionately larger
contributors of ionic Hg to the surrounding environment.
The influence of the Hg depositional environment on Hg speciation in mine
wastes is demonstrated by the consistent presence of Hg-chloride species in
samples collected at hot-spring Hg deposits, and their relative absence in
samples collected at silica-carbonate Hg deposits. Some exceptions exist, such
as the presence of eglestonite in the New Idria sample, although this could be
due to overprinting of a silica-carbonate Hg deposit with later hot-spring
hydrothermal systems. Nevertheless, based on the range of samples analyzed, a
general distinction in Hg speciation can be observed, with the presence of
Hg-chloride species as the primary difference between the two deposit types.
This implicates hot-spring Hg deposits as sites of higher priority for
remediation and introduces a new level of sophistication for such
prioritization, as speciation can now be considered alongside total Hg
concentration.
Effect of Ore Roasting on Hg Speciation
| |
|
 |
|
|
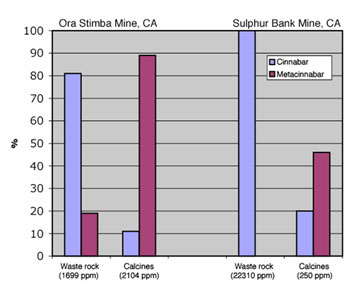
Figure 6.
Hg speciation of calcine and waste rock samples from two separate mines,
showing a distinct shift between the proportions of cinnabar (HgS, hex) and
metacinnabar (HgS, cub) as a result of roasting.
|
|
As seen, Hg-sulfides in the form of cinnabar and metacinnabar constitute the
highest proportion of Hg-containing species in Hg mine wastes, consistent with
the fact that cinnabar is the primary ore mineral in Hg deposits. However, the
proportions of metacinnabar detected in several roasted samples (Figure 5),
sometimes as high as 58%, are much greater than anticipated based on field
studies of Hg ore deposits, which only rarely report minor amounts of
metacinnabar. The transformation of cinnabar to metacinnabar during the
roasting process is a likely explanation for the elevated metacinnabar levels
detected in these calcines. Figure 6 shows Hg speciation results for
Hg-bearing mine wastes from the Sulphur Bank and Ora Stimba mines in
California. At both mines, a calcine pile and (unroasted) waste rock pile were
sampled to assess potential effects of ore roasting on Hg speciation. The
results show that cinnabar is the dominant Hg species in the unroasted waste
rock samples (present in proportions from 81-100%), while metacinnabar is more
prevalent in the calcines (46-81%). This suggests that ore roasting converts
cinnabar to metacinnabar by heating it above the cinnabar-metacinnabar
inversion temperature of 345° C (11). During the
mining process, Hg ore was crushed and then roasted in excess of 600°C to
release Hg as volatile elemental Hg° gas (12),
thereby providing temperatures sufficient to cause the conversion of
non-released cinnabar to metacinnabar. Such a process may also have introduced
impurities such as Fe, Se, and Zn that impeded the conversion back to cinnabar
upon cooling and are more common to the metacinnabar
structure (13,14). The
higher solubility of metacinnabar compared with cinnabar indicates that
calcines are of greater concern for cleanup than waste rock.
Effect of Particle Size on Hg Speciation
Particle size can exhibit a governing influence on factors such as the
concentration, speciation, solubility, and mobility of heavy metals in
contaminated wastes. The dependence of total Hg concentration and speciation on
particle size in Hg mine wastes was explored by collecting samples from
different Hg mines and separating them by dry sieving into a number of discrete
size fractions. Hg speciation of selected size fractions was then determined
using EXAFS spectroscopy (15).
| |
|
 |
|
|
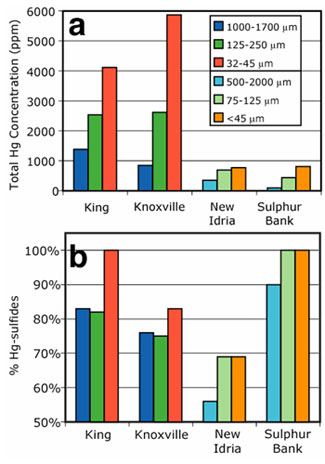
Figure 7.
(a) Total Hg concentration of various sieved size fractions from different Hg
mine calcines as determined by cold vapor atomic fluorescence spectroscopy
(CVAFS). (b) Percent Hg-sulfides as determined by EXAFS spectroscopy of
various sieved size fractions from different Hg mine calcines.
|
|
Hg total concentration and selected speciation results of these size fractions
are shown in Figure 7. As particle size decreases within each bulk sample the
total Hg concentration increases (Figure 7a), in some cases by nearly an order
of magnitude (e.g., a calcine from the Sulphur Bank mine shows an
increase in Hg concentration from 97 ppm in the 500-2000 µm size fraction
to 810 ppm in the <45 µm size fraction). This trend agrees with other
studies (16,17) which showed
elevated concentrations of Hg-sulfides in very fine sand and silt fractions
among Hg-mine wastes in northern California and Alaska. In addition to total
Hg concentration, EXAFS analysis reveals that the relative proportions of
Hg-sulfides (cinnabar, metacinnabar, and corderoite) in the sieved fractions of
calcines also increase with decreasing particle size (Figure 7b), although this
trend is less pronounced than that of total Hg concentration, increasing
between 8-18% among the samples studied.
These trends may be linked to the low solubility and hardness of cinnabar and
metacinnabar. As Hg-sulfides feature Ksp values in the range of 10-36
(18) and hardness levels of 2.5-3 (19), physical weathering (i.e. abrasion, fracturing, and
disaggregation) may exceed chemical weathering, (i.e. dissolution) under
certain conditions; furthermore, Hg-sulfides should weather at more rapid rates
than the quartz, silicates, and metal oxides that comprise the bulk of most
sample matrices. Mechanical weathering therefore likely yields the observed
enrichment in total Hg as particle size decreases. Additionally, with
decreasing particle size the increased available surface area of soluble Hg
minerals such as Hg-chlorides, oxides, and sulfates would facilitate the
dissolution of these species, resulting in the greater proportions of
relatively insoluble Hg-sulfides observed in the finest-grained fractions. A
study of Hg speciation in stream sediments of the Almadén cinnabar mining
region in Spain described a similar relationship between the proportion of Hg
present as Hg-sulfides and total Hg concentration (20). The combined results
of these studies indicate that these trends are common in cinnabar mine
environments.
The interested reader is referred to a comprehensive review of Hg speciation in
mining environments recently published by C. Kim (21) for more information and
details about the EXAFS speciation technique.
Primary Citation:
C. S. Kim, J. J. Rytuba and G. E. Brown, Jr., "Geological and
Anthropogenic Factors Influencing Mercury Speciation in Mine Wastes: an EXAFS
Spectroscopy Study", Appl. Geochem. 19, 379 (2004)
References:
-
A. L. Foster, G. E. Brown, Jr., T. Tingle, G. A. Parks, Am. Mineral. 83, 553-568 (1998).
-
K. S. Savage, T. N. Tingle, O. D. PA, G. A. Waychunas, D. K. Bird, Appl.
Geochem. 15, 1219-1244 (2000).
-
J. D. Ostergren, G. E. Brown, Jr., G. A. Parks, T. N. Tingle, Environ.
Sci. Technol. 33, 1627-1636 (1999).
-
G. Morin, J. D. Ostergren, F. Juillot, P. Ildefonse, G. Calas, G. E. Brown,
Jr., Am. Mineral. 84, 420-434 (1999).
-
A. Manceau, M. C. Boisset, G. Sarret, J. L. Hazemann, M. Mench, P. Cambier, R.
Prost, Environ. Sci. Technol. 30, 1540-1552 (1996).
-
A. Manceau, B. Lanson, M. L. Schlegel, J. C. Harge, M. Musso, L. EybertBerard,
J. L. Hazemann, D. Chateigner, G. M. Lamble, Am. J. Sci.
300, 289-343 (2000).
-
F. Juillot, G. Morin, P. Ildefonse, T. P. Trainor, M. Benedetti, L. Galoisy, G.
Calas, G. E. Brown, Jr., Am. Mineral. 88, 509-526 (2003).
-
C. S. Kim, G. E. Brown, Jr., J. J. Rytuba, Sci. Total Environ.
261, 157-168 (2000).
-
C. S. Kim, N. S. Bloom, J. J. Rytuba, G. E. Brown, Environ. Sci.
Technol. 37, 5102-5108 (2003).
-
J. J. Rytuba, W. R. Miller, USGS Circular C1103-A, 90-91 (1994).
-
G. Kullerud, Carnegie I. 64, 194-195 (1965).
-
J. J. Rytuba, Sci. Total Environ. 260, 57-71 (2000).
-
F. W. Dickson, G. Tunell, Am. Mineral. 44, 471-487 (1959).
-
V. L. Tauson, V. V. Akimov, Geochim. Cosmochim. Acta 61,
4935-4943 (1997).
-
C. S. Kim, J. J. Rytuba, G. E. Brown, Jr., Applied Geochemistry
19, 379-393
(2004).
-
J. B. Harsh, H. E. Doner, J. Environ. Qual. 10, 333-337 (1981).
-
H. Nelson, B. R. Larsen, E. A. Jenne, D. H. Sorg, Science 198, 820-824 (1977).
-
G. Schwarzenbach, M. Widmer, Helv. Chim. Acta 46, 2613-2628 (1963).
-
C. Klein, C. S. Hurlbut, Jr., Manual of Mineralogy (John Wiley & Sons, Inc.,
New York, NY, ed. 20th, 1985).
-
R. C. R. Martin-Doimeadios, J. C. Wasserman, L. F. G. Bermejo, D. Amouroux, J.
J. B. Nevado, O. F. X. Donard, J. Environ. Monit. 2, 360-366
(2000).
-
C. S. Kim, in Mercury: Sources, Measurements, Cycles and Effects. J. B.
Percival, Ed. (Mineral. Assoc. Canada, Short Course, 2005), vol. 34, pp.
95-122.
|
| PDF Version | | Lay Summary
|
| Highlights
Archive |
|
SSRL is supported by the Department of Energy, Office of Basic Energy Sciences.
The SSRL Structural Molecular Biology Program is supported by the Department of
Energy, Office of Biological and Environmental Research, and by the National
Institutes of Health, National Center for Research Resources, Biomedical
Technology Program, and the National Institute of General Medical Sciences.
|
|

 Christopher S.
Kim,1 James J. Rytuba,2 Gordon E. Brown, Jr.3
Christopher S.
Kim,1 James J. Rytuba,2 Gordon E. Brown, Jr.3






