
 Mary Corbett1, Yilin Hu3, Aaron Fay3,
Markus Ribbe3, Britt Hedman2, Keith
Hodgson1,2
Mary Corbett1, Yilin Hu3, Aaron Fay3,
Markus Ribbe3, Britt Hedman2, Keith
Hodgson1,2
1Department of Chemistry, and 2Stanford Synchrotron Radiation Laboratory, Stanford University; 3Department of Biochemistry and Molecular Biology, University of California, Irvine
Nitrogenase catalyzes the essential reaction in which atmospheric nitrogen is
converted into a form accessible for metabolic consumption (recently reviewed
in (1)). It has served as a focal point in the field of
bioinorganic chemistry both because of its important role in fixing nitrogen
under ambient conditions-the biological process accounts for ~ 50% of annual
production and does not require the extremes of heat and pressure utilized by
the industrial equivalent Haber-Bosch process-and because of the complexity of
its associated metalloclusters. Nitrogenase enzyme systems minimally consist of
two metalloproteins, a catalytic component and a specific reductase, which, in
the standard system, are referred to as the MoFe protein and the Fe protein. At
the active site of the MoFe protein is a heterometallic cluster, the
iron-molybdenum cofactor (FeMoco) (see Fig. 1, left). To better
understand the function of FeMoco and to provide insight into a route for the
chemical synthesis of a similar molecule, there is growing interest in
understanding how FeMoco is synthesized in vivo.
Fig. 1 Fourier transforms calculated over the k-range 2-16
Å-1 of EXAFS data
(dotted) and fits (solid) from (left) MoFe protein-bound FeMoco and
(right) the
NifEN-bound FeMoco precursor. The EXAFS data were obtained, respectively, by
subtraction of DnifB MoFe protein EXAFS
from wild-type MoFe protein EXAFS and
DnifB NifEN EXAFS from NifEN EXAFS.
The XAS results for the NifEN-bound FeMoco precursor are consistent with an
iron- and sulfur-containing cluster having at least seven iron atoms, the same
long-range order as FeMoco, and a similar average iron site geometry as FeMoco.
This precursor is formed in the absence of the several genes known to play a
role in FeMoco assembly and MoFe protein maturation, which suggests a larger
than expected role for the NifB protein in the assembly process. The most
likely trajectory for FeMoco biosynthesis from this state would be loss of the
non-sulfur-ligated terminal iron atom followed by incorporation of molybdenum
in its place. This simple metal substitution reaction would be the most likely
mechanism for the assembly of the alternative vanadium-containing nitrogenase
cofactor as well.
These EXAFS results provide much needed physical evidence to support a
mechanism for FeMoco biosynthesis and may prove instrumental in the development
of a strategy for the chemical synthesis of FeMoco and future opportunities for
a detailed analysis of this important yet enigmatic cluster.
Primary Citation
Corbett, M. C., Hu Y., Fay, A. W., Ribbe, M. W., Hedman, B., & Hodgson, K. O.
(2006) Structural insights into a protein-bound iron-molybdenum cofactor
precursor. Proc. Natl. Acad. Sci. USA 103, 1238-1243.
References
Genetic studies have shown that FeMoco is assembled
outside the MoFe protein in a stepwise process requiring several components
including NifB-co, an iron- and sulfur-containing FeMoco precursor, and NifEN,
an intermediary assembly protein where NifB-co is presumably converted to
FeMoco (2,3). Extended x-ray
absorption fine structure (EXAFS) spectroscopy experiments conducted at SSRL by
M. Corbett, B. Hedman, and K. Hodgson, in collaboration with M. Ribbe and
co-workers at UC Irvine, have recently been used to analyze the structure of a
NifEN-bound FeMoco precursor. This protein-bound precursor is found to be a
molybdenum-free analog of FeMoco (see Fig. 1, right), indicating that
the iron core of FeMoco is completely assembled early in the biosynthetic
pathway.
Both MoFe protein and NifEN contain two different types of iron-sulfur
clusters. In order to isolate the Fe K-edge EXAFS signal from FeMoco or its
precursor, EXAFS data were collected from the wild-type proteins and from
mutant proteins in which the cluster of interest is absent. Subtraction of the
EXAFS signal of the mutant proteins from that of the wild-type proteins in
ratios dependent on their respective metal contents produced data sets
representative of either MoFe-protein bound FeMoco or the NifEN-bound
precursor. Obtaining good data from this procedure required high resolution,
low noise EXAFS data-the collection of which was made possible by the high flux
and stability of focused wiggler beamline 9-3. The validity of this subtraction
technique was verified by the agreement of the XAS results for MoFe-protein
bound FeMoco with previous structural results (see, for example, 4).
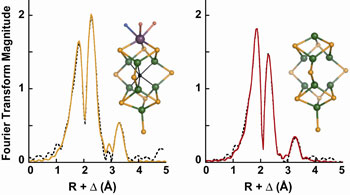
The insets show the structure of FeMoco (left) and a structural model
consistent with the XAS analysis of the NifEN-bound FeMoco precursor
(right).
Both structures were adapted from MoFe protein coordinates
(4) and are colored
by atom type: Mo (purple), Fe (green), S (orange), O (red), N (blue), and X
(black), which may be carbon, nitrogen, or oxygen.
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |