
 Xueyong Zhu1, Tobin J. Dickerson2,3, Claude J.
Rogers2,3, Gunnar F.
Kaufmann2,3,
Jenny M. Mee2,3, Kathleen M. McKenzie2,3, Kim D.
Janda2,3,4,* and Ian A.
Wilson1,4,*
Xueyong Zhu1, Tobin J. Dickerson2,3, Claude J.
Rogers2,3, Gunnar F.
Kaufmann2,3,
Jenny M. Mee2,3, Kathleen M. McKenzie2,3, Kim D.
Janda2,3,4,* and Ian A.
Wilson1,4,*
Departments of Molecular Biology1 and Chemistry2 and Immunology3, and The Skaggs Institute for Chemical Biology4, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037, USA.
Cocaine is a powerful addictive stimulant that affects the brain, and abuse of
cocaine has been a substantial social problem. Unfortunately, no FDA-approved
treatments exist for cocaine abuse, addiction, and overdose. Development of
effective treatment for cocaine abuse has been frustrated by the complex
neurochemistry in inhibiting a blocking agent. Nevertheless, within the past
decade, immunotherapy for cocaine abuse has been evaluated in pre-clinical and
human clinical trials.
Cocaine-binding antibodies have shown some promise in neutralizing the cocaine
toxicity, but they would be saturated by high concentrations of cocaine because
of the 1:1 binding stoichiometry. A cocaine-degrading catalytic antibody, such
as 7A1, is far more effective in metabolizing the drug, since the antibody
would be regenerated with each turnover1. Catalytic antibodies have emerged as
a powerful tool at the interface of chemistry and biology. In this regard,
antibody-catalyzed ester hydrolysis is one of the hallmark reactions. Since
cleavage of the benzoate ester of cocaine produces the nonpsychoactive
metabolites ecgonine methyl ester and benzoic acid, it is an excellent target
for an immunopharmacological strategy. A crystal structure of the mouse
monoclonal antibody 7A1 would help to determine the molecular basis for
catalysis and provide a foundation for murine antibody humanization and
mutagenesis studies to increase the catalytic proficiency for potential
therapeutic use.
 Figure 1 Crystal structure of the 7A1 Fab' cocaine complex with the
secondary structure of the antibody light (L) and heavy (H) chains colored in
cyan. Substrate cocaine is also shown in spheres with yellow carbons, blue
nitrogen, and red oxygens in the active site.
Figure 1 Crystal structure of the 7A1 Fab' cocaine complex with the
secondary structure of the antibody light (L) and heavy (H) chains colored in
cyan. Substrate cocaine is also shown in spheres with yellow carbons, blue
nitrogen, and red oxygens in the active site.
|
Although it is rarely possible to acquire experimentally-determined structures
of each step along an enzyme reaction coordinate, 7A1 Fab' antigen binding
fragment was able to be co-crystallized with substrate, a transition state
analog (TSA), both products and heptaethylene glycol. Using x-ray diffraction
data collected at SSRL beamline 9-2 and the ALS, 7A1 Fab' and six complexes
with substrate cocaine (Figure 1 and 2b), TSA, both products (ecgonine methyl
ester and benzoate), one product ecgonine methyl ester, and finally the other
product benzoate, as well as heptaethylene glycol, were determined at 1.5-2.3 Å
resolution (Figure 2). Here, high resolution snapshots are presented for the
complete reaction cycle of the cocaine catalytic antibody.
Significant conformational changes were observed along the 7A1-catalyzed
cocaine hydrolysis pathway, but are generally limited to some active site key
residues and ligands themselves. Antibody CDR loop movements (up to 2.3 Å) and
large side-chain movements (up to 9 Å) alter the antibody active site from
"open" to "closed" to "open" (with the approximate size changes from 320 to 500
Å3) for the substrate, transition state and product states, respectively.
In the unliganded apo 7A1, the active site adopts an "open" form with two
essential residues TyrL94 and TyrH97 showing flexible side-chains (Figure 2a).
In the substrate cocaine-bound state, TyrH97 is fully ordered, TyrL94 displays
some partial occupancy to accommodate the two side-chain rotamers of TrpH47,
while the active site retains a modified "open" form (Figure 2b). In the
transition state analog complex, the active site shows the "closed" form with
the CDR loops, particularly H2, moving towards the active site, along with the
rearrangements of side chains of ArgH52, ArgH58 and IleH56 by several angstroms
(Figure 2c); TyrL94 and TyrH50 now hydrogen bond with the pro-R phosphonate
oxygen of the TSA, and possibly constitute an oxyanion hole to stabilize the
transition state. When the cocaine hydrolysis is achieved, the active site
returns to an intermediate "open" with two products initially remain trapped;
the side chains of TyrH50, ArgH52, ArgH58 and IleH56 adopt conformations
between those found in the transition state and those in the substrate-bound
state, and the side chain of TyrL94 has partial stabilization and a different
rotamer (Figure 2d). From this comprehensive series of crystal structures, a
catalytic mechanism has been proposed, as well as possible mutations that
explore how to improve catalytic proficiency.
This work was supported by the National Institutes of Health grants GM38273
(IAW), DA08590 and DA15700 (KDJ), and The Skaggs Institute for Chemical
Biology, The Scripps Research Institute (IAW and KDJ).
Primary Citation
Zhu, X., Dickerson, T.J., Rogers, C.J., Kaufmann, G.F., Mee, J.M., McKenzie,
K.M., Janda K.D. and Wilson I.A. Complete reaction cycle of a cocaine catalytic
antibody at atomic resolution. Structure, 14, 205-216 (2006).
References
Scripps Press Release: http://www.scripps.edu/news/press/020706.html
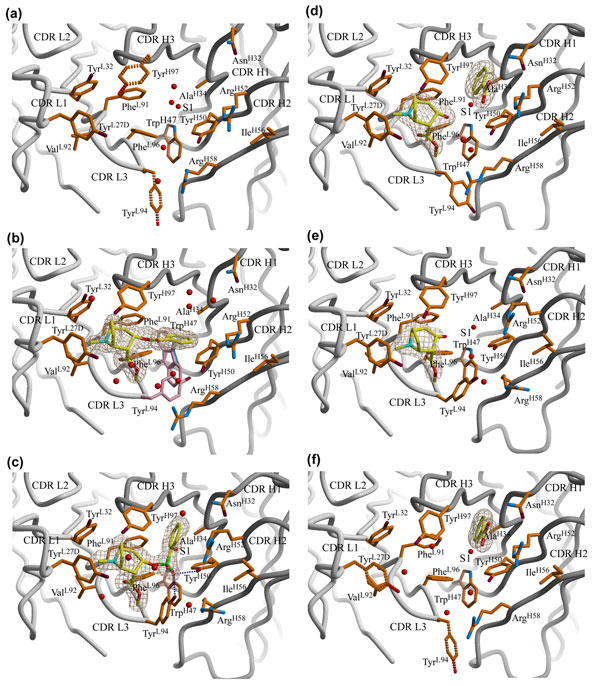
Figure 2 The active site of 7A1 corresponding to different steps in the
reaction pathway. (a) Apo form (1.5 Å resolution). (b) Complex with substrate
cocaine (1.5 Å). (c) Complex with transition state analog (TSA) (1.85 Å). (d)
Complex with both products ecgonine methyl ester and benzoate (2.1 Å). (e)
Complex with product ecgonine methyl ester (2.3 Å). (f) Complex with product
benzoate (1.85 Å). Disordered side chains are highlighted in dashed bonds.
Alternate conformations of TrpH47 and the partially-occupied TyrL94 are
rendered with pink side chains in the 7A1 Fab' cocaine complex. The
corresponding ligands for each structure are shown with yellow carbons, blue
nitrogens, red oxygens and green phosphorus atoms. Water molecules in the
active site are shown in red spheres and the conserved water is labeled S1
(From Zhu et al., 2006).
Related Links
Wilson Research: http://www.scripps.edu/mb/wilson
Janda Research: http://www.scripps.edu/chem/janda
| SSRL is supported by the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by the Department of Energy, Office of Biological and Environmental Research, and by the National Institutes of Health, National Center for Research Resources, Biomedical Technology Program, and the National Institute of General Medical Sciences. |