Stanford Synchrotron Radiation Laboratory
Stanford Linear Accelerator Center
|
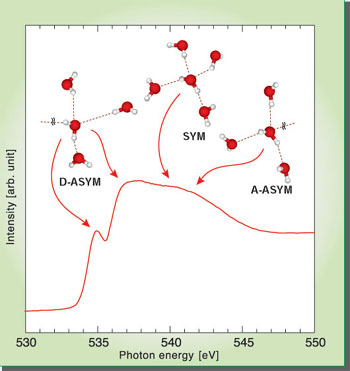
Oxygen K-edge X-ray absorption of liquid water in the presence of dissolved ions.
We
have studied the electronic structure of liquid water by using X-ray
Absorption Spectroscopy (XAS) at the oxygen K-edge. Since the X-ray
absorption process is faster than a femtosecond, it allows probing
of the molecular orbital structure of frozen, local geometries of
water molecules at a time-scale that has not previously been accessible.
The results indicate that the electronic structure of liquid water
is significantly different from that of the solid and gaseous forms,
resulting in a pronounced pre-edge feature below the main absorption
edge in the spectrum. Theoretical calculations of these spectra
suggest that this feature originates from specific configurations
of water, for which the H-bond is broken on the H-donating site
of the water molecule. By using the same approach I also study the
chemical bonding of water in the first hydration sphere to transition
metal ions in aqueous solutions. The experimental technique is for
the first time applied to the study of the oxygen K-edge absorption
of liqu
id water in the presence of dissolved ions.
|