__________________________________________________________________________
SSRL Headlines Vol. 12, No. 5 - November 2011
__________________________________________________________________________
Contents of this Issue:
__________________________________________________________________________
1.
SSRL User Operations Resume
(SLAC News Center article by Lori Ann White)
Stanford Synchrotron Radiation Lightsource users arrived back at SLAC last week as the 2011–2012 run gets under way. They returned to an SSRL that can offer both facility-wide improvements and upgraded equipment on some beam lines.
For SSRL as a whole, the watchword is "beam stability." Considerable effort has gone into providing more stable electron beams, which translate into more consistent x-rays and more accurate measurements for researchers in the experimental hutches. One problem tackled over the shutdown is the effect of daily temperature fluctuations on the SPEAR3 accelerator.
Read more in SLAC Today...
2. Science Highlight —
Mystery Solved: Nitrogenase's Central Atom is Carbon
(contact: Serena DeBeer, serena.debeer@mpi-mail.mpg.de)
|
 |
If we could make plant food from nitrogen the way nature does, we would have a much greener method for manufacturing fertilizer;
the current industrial process requires such high temperatures and pressures that it consumes about 1.5 percent of the world's energy.
Now scientists working at the Stanford Synchrotron Radiation Lightsource have taken an important step toward understanding how nature performs this
reaction, by establishing the nature of a key atom that researchers had sought
to identify for more than a decade.
The atom lies at the heart of the active component of an enzyme called nitrogenase, which plays a critical role in converting nitrogen in the air into a form that living things can use:
ammonia. Although the structure of this enzyme has been known for a long
time, scientists have long sought to determine if this atom, which lies in
the center of the metal cluster where the ultimate action takes place, is
carbon, nitrogen or oxygen; among other things, they hope to eventually reverse-engineer it and mimic nature's gentle version of the reaction.
"The fascination with this enzyme is the fact that it enables this reaction to take place at room temperature and atmospheric pressure," said chemist Serena DeBeer of Cornell University and the Max Planck Institute for Bioinorganic Chemistry, who led the team that performed crucial experiments at
SSRL. So hot was the race to identify the mystery atom that it ended in a photo finish: in the November 18 issue of
Science, two independent teams, using different approaches, identify the atom as carbon.
Read the full SLAC press release...
To learn more about this research see the
full scientific highlight.
3.
Science Highlight
— Researchers Determine Structure of Key DNA Transcription Molecule
(contact: Yuichiro Takagi, ytakagi@iupui.edu)
|
 |
Scientists have deciphered the structure of an essential part of Mediator, a complex molecular machine that plays a vital role in regulating the transcription of DNA. In the course of cellular operations, signals are sent to each cell's DNA asking that some genes be activated and others be shut down. The Mediator transcription regulator accepts and interprets those instructions, telling RNA polymerase II where and when to begin copying sections of the DNA.
Mediator is a gigantic molecular machine composed of 21 proteins organized into three modules known as the head, the middle, and the tail. Using x-ray crystallographic data collected at the Stanford Synchrotron Radiation Lightsource and the Advanced Photon Source, the research team, led by scientists from the Indiana University School of Medicine, was able to describe in detail the structure of the Mediator Head module, the most important for interactions with RNA polymerase II.
One immediate benefit of the research will be to provide detailed mapping of previously known mutations that affect the regulation of the transcription process. In addition, the ability to solve such complex structures will be important because multi-protein complexes such as Mediator will most likely become a new generation of drug targets for treatment of disease.
To learn more about this research see the
full scientific highlight.
4.
Science Highlight — Structure and Reactivity of a Non-Heme Iron Complex
(contacts: Samuel Wilson, s4wilson@stanford.edu, and Edward I. Solomon, edward.solomon@stanford.edu)
|
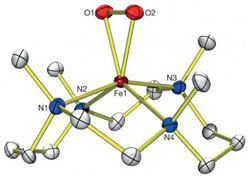 |
The life-sustaining element oxygen can’t do its job alone. Specialized enzymes, containing metallic elements including iron, cause O2 to split into two separate oxygen atoms. In this form, oxygen can react with other biological molecules. The precise mechanism of oxygen activation by iron complexes has long eluded researchers, in part because the reaction—which proceeds through multiple intermediate stages—happens in mere fractions of a second.
Recently, a team of researchers from Stanford, SSRL, and Ewha Womans University used the Stanford Synchrotron Radiation Lightsource to capture all three of the intermediate structures that one iron complex morphs into as it cleaves the O2 bond—including one intermediate that exists for less than 2 milliseconds before converting into a different form.
This is the first time researchers have so fully characterized this type of iron- and oxygen-containing molecule, which is called non-heme iron because it lacks the heme group for which the iron-containing molecule hemoglobin that carries oxygen in red blood cells is named. The newly discovered reaction mechanism could help scientists better understand diseases of non-heme iron enzymes, such as phenylketonuria. The disease, which prevents breakdown of the amino acid phenylalanine, can cause developmental defects in babies and lasting health problems for adults. Other more distant applications may affect energy production and industrial processes that use similar chemistry.
To learn more about this research see the
full scientific highlight.
5.
A Warm Welcome to Newly Elected SSRL UEC Members
The votes are in! New members of the SSRL Users' Organization Executive Committee have been elected. For the next three years, Alberto Salleo (Stanford University) will serve as the Materials Science Representative; Juana Rudati (Xradia) as the Industry Representative; Eva Rose Balog (UC Santa Cruz) as a Graduate Student Representative; and Rodrigo Noriega (Stanford University) as a Graduate Student Representative.
In addition, Sarah Hayes has been elected Vice Chair of the SSRL UEC. In this position, Sarah will play an important role in co-organizing the 2012 Users' Conference and will take over from current UEC Chair Serena DeBeer in FY2013.
We extend a warm welcome to our incoming representatives and many thanks to outgoing UEC members. We appreciate their willingness to represent the interests of their respective user communities in this capacity.
6. Latest SSRL Seismic Upgrade Completed
(SLAC News Center article by Mike Ross)
It was just a coincidence that a series of small earthquakes in Berkeley began on October 20—the very day of the statewide earthquake preparedness campaign, the "Great California Shake Out." But the timing surely heightened the satisfaction of SLAC crews and contractors, who had just completed the second phase of the project to upgrade the seismic stability of Stanford Synchrotron Radiation Lightsource structures.
SSRL structures suffered no damage during the last major earthquake to rock the Bay Area, on Oct. 19, 1989. The goal of the latest retrofit projects is to ensure that they withstand the jolts created by larger and closer earthquakes, including those on the section of the San Andreas Fault located just five miles west of SSRL.
Read more in SLAC Today...
7.
Announcements: SLAC Public Lecture, NESRC Computing Proposals, New SSRL Photo
SLAC Public Lecture. You're invited to join SSRL Staff Scientist Clyde
Smith for the public lecture "Chasing Super Bugs with Smarter Drug Design,"
to be presented at 7:00 p.m. on Tuesday, November 29 in SLAC's Panofsky
Auditorium. For those who can't make it in the evening, he will present a
reprise at noon on Thursday, December 1 in SLAC's Kavli Auditorium. All are
welcome to attend. Learn more on the
public lecture website.
Call for NERSC Computing Proposals.
Researchers are invited to apply for an allocation of computer time and archival storage at the National Energy Research Scientific Computing (NERSC) Center if their research project is funded by or relevant to the DOE Office of Science mission. Proposals are due December 1; learn more on the NERSC website.
New Photo: SSRL at Dusk. Looking for a great photo of SSRL to spice up
your next presentation or poster? Look no further than
this new image
(also shown in the above article "SSRL User Operations Resume"), courtesy of SLAC Communications.
8.
User Administration Update: Beam Time Requests and Proposals Due December 1
(contacts: Cathy Knotts, knotts@slac.stanford.edu; Lisa Dunn, lisa@slac.stanford.edu)
X-ray/VUV Beam Time Requests for February through May 2011 beam time are due
Thursday, December 1. December 1 is also the next deadline for submitting new
X-ray, VUV and Macromolecular Crystallography proposals. Please submit your proposals and
requests via our
user portal.
9. In the News
— Ten Questions, Mystery Atom Cracked
Ten Questions for a Beamline Scientist: Apurva Mehta
Fifteen years ago, Apurva Mehta volunteered to help a friend build beamline parts at SSRL. Today, he's "still mucking around with beamlines."
In this Q&A, Apurva shares how he landed at SLAC and his adventures in a wide range of projects, from advanced semiconductors to ancient Greek pottery.
See full Q&A at energy.gov...
Enzyme Critical for Life, X-ray Emission Cracks Mystery Atom
Like a shadowy character just hidden from view, a mystery atom in the middle of a complex enzyme called nitrogenase had long hindered scientists' ability to study the enzyme fully.
But now an international team of scientists has pulled back the curtain using powerful synchrotron spectroscopy and computational modeling to reveal carbon as the once-elusive atom.
See full story at physorg.com...
__________________________________________________________________________
SSRL Headlines is published electronically monthly to inform SSRL users,
sponsors and other interested people about happenings at SSRL. SSRL is a
national synchrotron user facility operated for the U.S.
Department of Energy Office of Basic Energy Sciences by Stanford
University. Additional support for
the structural biology program is provided by the DOE
Office of Biological and Environmental Research, the NIH
National Center for Research Resources and the NIH National Institute of General Medical
Sciences. Additional information about
SSRL and its operation and schedules is available from the SSRL website.
__________________________________________________________________________
Unsubscribe
To leave the SSRL-HEADLINES distribution, send email as shown
below:
To: LISTSERV@SSRL.SLAC.STANFORD.EDU
Subject: (blank, or anything you like). The message body should read
SIGNOFF SSRL-HEADLINES
That's all it takes. (If we have an old email address for you
that is forwarded to your current address, the system may not recognize who
should be unsubscribed. In that case please write to
ssrl-headlines-request@ssrl.slac.stanford.edu and we'll try to figure out who
you are so that you can be unsubscribed.)
Subscribe
If a colleague would like to subscribe to the list, he or she
should send To: LISTSERV@SSRL.SLAC.STANFORD.EDU and use the
message body SUBSCRIBE SSRL-HEADLINES
| Last Updated: |
21 NOVEMBER 2011 |
| Headlines Editor: |
Kelen Tuttle |
|





