A team of researchers from the Technical University of Denmark and the SUNCAT Institute at the SLAC National Accelerator Laboratory and Stanford University has demonstrated the superior performance of nanoparticles of platinum-yttrium (PtxY) as catalysts for oxygen electroreduction.
Polymer electrolyte membrane fuel cells (PEMFCs) hold the promise to potentially become zero-emission alternative power sources for automotive vehicles. Moreover, in comparison to batteries, they provide much longer driving ranges and faster refueling times. Even so, the widespread use of PEMFCs has been hampered by the need for a large amount of platinum catalyst at the cathode, where oxygen reduction takes place. Even with current state-of-the-art technology, it is too expensive to scale up PEMFC production to make a global impact.
Arguably, the most viable route towards decreasing the platinum loading is to employ alloys of platinum with other metals as catalysts for oxygen reduction. Most researchers have focused on alloys of platinum with late transition metals, such as nickel, cobalt, copper or iron. The catalytic activity of Pt atoms in these alloys was found to be much higher than in pure Pt. However, the long-term stability of these catalysts is typically compromised by their tendency to degrade via dealloying, resulting in the segregation of the less noble late transition metal to the surface of the catalyst, which subsequently dissolves in the acidic electrolyte.
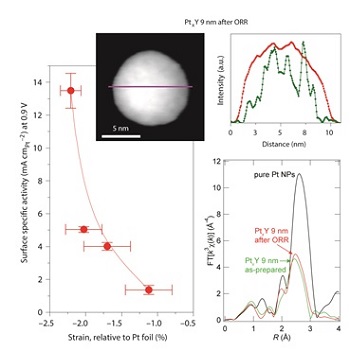
Figure: Scanning transmission electron microscopy energy-dispersive X-ray spectroscopy (STEM-EDX, top) showing the core-shell structure of PtxY nanoparticles after oxygen reduction reaction (ORR) test. The ORR activity is correlated with the average compressive strain determined by grazing-incidence extended x-ray absorption fine structure spectroscopy (EXAFS, bottom right).
In 2009, a team of researchers from the Technical University of Denmark discovered a new alloy for oxygen reduction, platinum-yttrium, which was more active and potentially more stable than other alloys of platinum.1 The exceptionally strong bond between platinum and yttrium should prevent dealloying. However, platinum-yttrium was first tested in bulk polycrystalline form. Yet, in a real-world fuel cell, the catalyst would have to be implemented in nanoparticulate form, i.e. with a high ratio of surface atoms to the catalyst core. This turned out to be a highly challenging task because platinum is a noble metal and can be easily reduced to the metallic form, whereas yttrium is highly oxophilic and hence extremely challenging to reduce.
The new study, published in Nature Chemistry, describes the synthesis of platinum-yttrium alloy nanoparticles for the first time. The researchers used a physical method that involved magnetron sputtering. The advantage of this synthesis route is that it allows a high degree of control over the nanocatalyst, which is produced with a well-defined size and composition. This, in turn, allows for scientific insights into the relationship between the structure and functionality of the particles.
The particles were characterized using a large number of spectroscopic and imaging techniques. The most active catalysts exhibited a high mass activity of 3.05 A/mg Pt – five times greater compared to commercial platinum catalysts. Moreover, the new catalyst retained 63% of its initial activity in an extended stability test.
It turns out that the catalyst forms a Pt overlayer at the surface, as the yttrium will leach out from the first nanometer of the catalyst surface. Extended x-ray absorption fine structure (EXAFS) measurements, carried out at SSRL’s Beam Line 11-2, showed that the alloy structure of the particle’s core induces compressive strain on the Pt overlayer. This, in turn, leads to improved catalytic activity for oxygen reduction, relative to pure platinum catalysts, due to a mildly weaker bond between the platinum surface atoms and the hydroxyl reaction intermediates.
The EXAFS experiments were performed under grazing incidence – a unique capability of SSRL’s Beam Line 11-2. This technique was essential to study extremely small amounts of nanoparticles that were deposited on a flat carbon substrate in order to avoid agglomeration.
In summary, the study represents a proof-of-principle that platinum-yttrium nanoparticles can be synthesized and are highly active for oxygen reduction. The physical origin of the activity enhancement has been identified: It is due to the formation of a core-shell structure with a compressively strained outer Pt shell. The promising material has the potential for an even better, record-setting performance if the particle size and composition can be further optimized. However, in order to find its way into fuel cell applications, researchers will have to address another challenge first: the development of a chemical synthesis method that enables catalyst production in large quantities.
1. J. Greeley et al., "Alloys of Platinum and Early Transition Metals as Oxygen Reduction Electrocatalysts", Nat. Chem. 1, 552 (2009)
P. Hernandez-Fernandez, F. Masini, D. N. McCarthy, C. E. Strebel, D. Friebel, D. Deiana, P. Malacrida, A. Nierhoff, A. Bodin, A. M. Wise, J. H. Nielsen, T. W. Hansen, A. Nilsson, I. E. L. Stephens and I. Chorkendorff, "Mass-selected Nanoparticles of PtxY as Model Catalysts for Oxygen Electroreduction", Nat. Chem. (2014) doi: 10.1038/nchem.2001

