Uranium (U) is one of the most prevalent radionuclide contaminants in soils and groundwater across the world as a result of nuclear fuel production, weapons manufacturing, and research activities. The environmental risks posed by U are determined largely by the degree of its mobility, which strongly depends on redox conditions. Under oxic conditions, U(VI) is soluble and forms stable complexes with carbonate and calcium in groundwater. In contrast, reduced U(IV) species are often immobilized as sparingly soluble U(IV) solid phases such as uraninite (UO2) by biotic or abiotic redox processes.
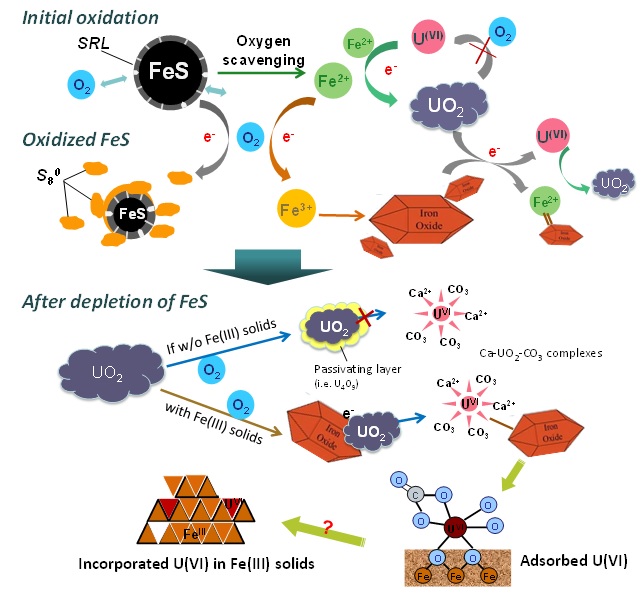
Naturally-occurring nanoscale iron sulfide (FeS) has been shown to supply electrons for abiotic uranium reduction in biostimulated aquifers. Reduced U(IV) forms uraninite (UO2) precipitates, which are relatively insoluble and less mobile than dissolved U(VI) in groundwater. The close association of UO2 with FeS suggests that FeS may serve as an effective redox buffer for long-term U stabilization in subsurface environments. Upon oxidant intrusion, FeS may therefore be expected to inhibit reoxidation of reduced-U solid phases formed during a reductive bioremediation phase. Understanding the role of FeS in protecting UO2 against reoxidation is therefore important for assessing the long-term stability of U-bioremediated contaminated aquifers.
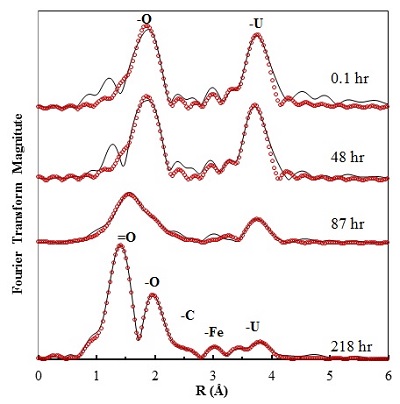
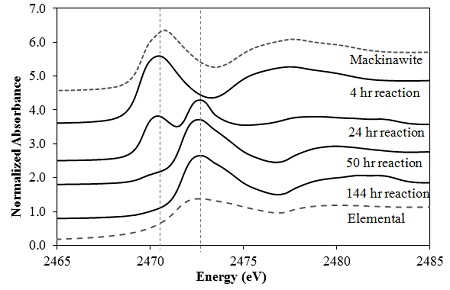
A recently published study in Geochimica et Cosmochimica Acta by researchers from the University of Michigan and the Pacific Northwest National Laboratory (PNNL), investigated the oxidative transformation of UO2 nanoparticles by oxygen in the presence of FeS under simulated groundwater conditions. In this study, a batch reactor was equipped with a water jacket for temperature control, and a multi-port lid for accommodating various probes, sampling, and gas mixtures with fixed partial pressures. The oxidation products and U speciation were monitored as a function of time in a strict abiotic system. The kinetic profiles of dissolved U showed that FeS inhibited UO2 dissolution by effectively scavenging oxygen and keeping dissolved-oxygen (DO) levels low. X-ray absorption spectroscopy (XAS) analysis based on data collected at SSRL’s Beam Line 11-2 was used to characterize the local molecular structure around the U core at low concentrations. As a result of FeS rapidly consuming DO, solid-phase U remained as U(IV)O2 until FeS was nearly completely oxidized (Fig. 1). The oxidation of structural S(-II) in FeS to elemental sulfur was found to be one of the major scavenging mechanisms for DO by FeS, as evidenced by measurements with the soft X-ray beam generated at Beam Line 4-3 (Fig. 2).
The oxidation of FeS also resulted in the formation of predominantly Fe(III) oxyhydroxides. Following the complete oxidation of FeS, oxidative dissolution of UO2 began. During fast reoxidation, the rate of UO2 oxidation was monitored and compared to the rate in control experiments in the absence of FeS and Fe(III) products. The presence of Fe(III) oxyhydroxide solids accelerated the oxidative dissolution of UO2 compared to the control. It was speculated that Fe(III) on the oxyhydroxide surface facilitated the electron transfer from U(IV) to oxygen. The subsequent adsorption of multinuclear U(VI)-carbonato complexes by nano goethite or lepidocrocite was revealed as a major retention mechanism for the dissolved U(VI) species formed during UO2 oxidation, with no evidence of U(VI) incorporation into the Fe(III) mineral structure (Fig.1).
These results confirm that the stability of reduced U(IV) solid phases is achieved in the presence of FeS nanoparticles as a result of the rapid scavenging of DO by FeS, before oxygen can react significantly with U(IV). FeS-associated U(IV), which has been observed following active bioreductive in situ treatment of U(VI)-contaminated groundwater [1], may be protected against oxidative dissolution for sustained periods of time under fluctuating redox conditions in subsurface environments.
This research was funded by Subsurface Biogeochemical Research Program in the Office of Science (BER), U.S. Department of Energy.
J. R. Bargar, K. H. Williams, K. M. Campbell, P. E. Long, J. E. Stubbs, E. I. Suvorova, J. S. Lezama-Pacheco, D. S. Alessi, M. Stylo, S. M. Webb, J. A. Davis, D. E. Giammar, L. Y. Blue and R. Bernier-Latmani, "Uranium Redox Transition Pathways in Acetate-amended Sediments", Proc. Natl. Acad. Sci. USA 110, 4506 (2013) doi: 10.1073/pnas.1219198110
Y. Bi, S. P. Hyun, R. Kukkadapu and K. F. Hayes, "Oxidative Dissolution of UO2 in a Simulated Groundwater Containing Synthetic Nanocrystalline Mackinawite", Geochim. Cosmochim. Acta 102, 175 (2013) doi: 10.1016/j.gca.2012.10.032

