Hydrogen gas (H2) is an energy source for a variety of microorganisms living on Earth today and is thought to have been an important source of energy on early Earth. Hydrogen is produced from the reaction of ultramafic and mafic rocks (rich in iron (Fe) and magnesium (Mg)) with anoxic water at temperatures >200°C. The mechanism responsible for H2 production is the transformation of reduced iron (Fe(II)) in primary minerals (e.g. olivine) to oxidized iron (Fe(III)) that is incorporated into secondary minerals, mainly magnetite (Fe3O4). Hydrogen generated at these high temperatures is known to support life at hydrothermal systems where the high-temperature water and gases are vented to the Earth’s surface (e.g. seafloor, terrestrial hotsprings). At these interfaces, the cooler temperatures allow for life, tolerant of temperatures only up to ~122°C, to survive.
Considering only these high-temperature reactions as a source of H2 ignores the potentially large volume of Earth’s crust that interacts with water at temperatures much lower than 200°C. At these temperatures, H2 produced from water-rock reactions could potentially support in-situ subsurface microbial communities. However, little experimental work has been done to constrain the amount and mechanism of H2 production at temperatures within the temperature limits of life. Thus, little is known about the potentially extensive subsurface microbial habitat that may be supported by H2 generated from low-temperature water-rock reactions.
Recently, a team of researchers from the University of Colorado – Boulder, Department of Geological Sciences and Laboratory for Atmospheric and Space Physics, and the University of Alaska – Fairbanks, Department of Chemistry and Biochemistry, combined powdered ultramafic rocks (peridotite) and minerals (olivine, pyroxene, magnetite) with anoxic water and incubated them at 55 and 100°C for approximately 100 days. The team monitored the gaseous and aqueous chemistry of the system over time and documented the production of significant amounts of H2 in experiments with 5 out of the 6 tested substrates.
The team employed x-ray absorption spectroscopy (XAS) at the SSRL microXAS imaging Beam Line 2-3 to investigate the nature of the solid phase reaction products. Using Fe K-edge XAS (~7 keV), the authors rapidly measured data that revealed the oxidation state, speciation and distribution of Fe-bearing phases on the small amount of microscale reaction products. The technique applied was the subject of a previous publication [1] as well as a previous August 2011 SSRL Highlight. Complementary data were collected at 11 keV to map the distribution of trace elements (e.g. chromium (Cr)).
The data revealed the preferential localization of Fe(III)-bearing reaction products on spinel minerals (e.g. magnetite and chromite (FeCr2O4)) that were minor components of the primary reaction substrates. Spinels are minerals with a cubic structure and the general formula M2+M3+2O4 (where M = metal, e.g. Fe, Cr). Spinels are often found as accessory minerals in basalts and partially altered peridotites, rocks which constitute the majority of the ocean crust. Spinels are highly conductive minerals known to transfer electrons to metals in both the solid and aqueous phase. Subsequently, the team found that the amount of H2 produced in the experiments generally increased with increasing volume percent of spinels in each substrate.
The observations made at BL 2-3 were a key component to the new hypothesis proposed by the team to explain the mechanism of H2 production from low-temperature water-rock reactions. This hypothesis proposes that H2 production is promoted by the transfer of electrons occurring at the surface of spinel mineral phases. Spinel phases that possess Fe(II) in their mineral structure may transfer electrons from those structural Fe(II) atoms to water (H2O) or protons (H+) sorbed to the mineral surface, producing H2 gas. Further, Fe(II) in the aqueous phase from the dissolution of primary silicate phases (e.g. olivine ((Mg,Fe)2SiO4), pyroxene (Ca(Mg,Fe)Si2O6)) may act as an external source of electrons. Fe(II) can sorb to the surface of the spinel minerals and transfer electrons to the spinel which, in turn, transfers electrons to water (H2O) or protons (H+), producing H2 gas.
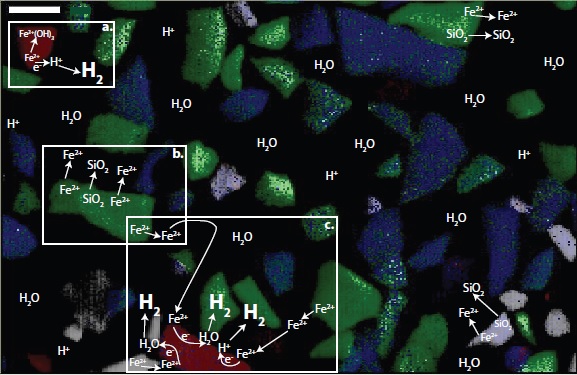
The authors propose that spinel surface-promoted electron transfer is an important mechanism leading to the production of H2 during low-temperature water-rock reactions. Eventually, H2 production ceases when the spinels are passivated by the formation of Fe(III)-bearing secondary mineral layers. In low-temperature oceanic and terrestrial aquifers spinel phases may play an important role in H2 production. Continued exposure of fresh rock surfaces from hydration and cracking of the rocks could lead to sustained H2 production with time. Thus, H2 produced from low-temperature water-rock reactions may be able to support subsurface H2-utilizing microbial communities. Furthermore, spinel-bearing rocks, such as basalts, are common on the surface of Mars and minerals diagnostic of the occurrence of water-rock reactions have also been detected on Mars’ surface. Perhaps spinels could play a role in supporting putative microbial habitats on other terrestrial planetary bodies.
This work was conducted at the Stanford Synchrotron Radiation Lightsource (SSRL), a national user facility operated by Stanford University on behalf of the Department of Energy, Office of Basic Energy Sciences. The SSRL Structural Molecular Biology Program is supported by DOE Office of Biological and Environmental Research and the National Institutes of Health.
- L. E. Mayhew, S. M. Webb, and A. S. Templeton,“Microscale Imaging and Identification of Fe Speciation and Distribution during Fluid–Mineral Reactions under Highly Reducing Conditions”, Environ. Sci. Technol. 45, 4468 (2011) doi: 10.1021/es104292n
L. E. Mayhew, E.T. Ellison, T.M. McCollom, T.P. Trainor, and A. S. Templeton, “Hydrogen Generation from Low Temperature Water-Rock Reactions”, Nat. Geosci. 6, 478 (2013) doi:10.1038/ngeo1825

