Olefins are the basic building blocks for many products of the petrochemical industry and are currently produced by steam cracking of naphtha or ethane. Increasing oil and gas prices are driving the industry toward producing olefins by the Fischer-Tropsch process instead, using syngas (CO + H2) derived from cost-advantaged feedstocks, such as natural gas, biomass and coal.
The Fischer-Tropsch-to-Olefins (FTO) process commonly uses an iron-based catalyst, where the addition of promoters can improve catalytic activity and affect selectivity toward light olefins. The active sites are thought to be located on metal nanoparticles, but their exact chemical nature is still under debate. In this respect, recent developments in advanced in situ spectroscopic techniques have proven to be very important for studying the catalyst under working conditions, providing essential insight into structure-performance relationships. Within this scenario, full-field Transmission hard X-ray Microscopy (TXM) appears to be an excellent characterization method because of its potential to image nanoscale features of relatively thick objects (tens of microns), combining chemical and morphological sensitivity. Therefore, we have used the TXM technique combined with a specially designed reactor in order to perform operando characterization during catalysis, under full reaction conditions, including temperature and pressure.
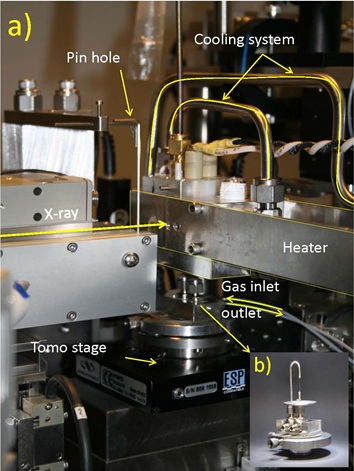
Scientists from Utrecht University (I. Gonzalez, K. Cats, F.M.F. de Groot and B.M. Weckhuysen), The Dow Chemical Company (T. Davidian, M. Ruitenbeek) and Fondazione Bruno Kessler (F. Meirer), in close collaboration with SSRL (J.C. Andrews, Y. Liu, J. Nelson and P. Pianetta), have designed a reactor which allows treatments of catalytic solids at 10-30 bar and up to 600 οC under gas flow. The reactor consists of a quartz capillary (100 µm diameter, 10 µm wall thickness) mounted on the sample stage with x, y, z and θ motion. Moreover, it is also possible to perform tomography studies under in situ conditions because of the special heater customized and manufactured at SSRL (Figure 1). Using this special reactor system it is possible to follow a single nanoscale catalytic particle during activation and under Fischer-Tropsch reaction conditions. Acquiring a stack of images at different energies across the Fe X-ray absorption K-edge generates full spectral information at every pixel, which can be used to image and distinguish specific chemical species at tens of nm resolution [1]. The combination of chemical information with high spatial resolution is valuable when the system under study has a marked dynamic character in the active phases, as is the case for Fe-based catalysts for FTO.
![Figure 2 (a) Snapshots of 3D elemental mapping [2] (tomography acquired above and below Fe and Zn K-edges) of a single fresh FTO catalyst particle (19.5 µm diameter), with Fe in red (for all Fe: Fe2O3, Fe3O4 and Fe2TiO5), Zn in green (for ZnO) and Ti + K in white/yellow/orange (for TiO2 and K2O; white used for the highest concentration). (b) 2D chemical composite maps [1] showing the spatial distribution of different iron species over an area 21 x 21 μm2. For chemical maps of the fresh catalyst and after 4 hours under reaction conditions (10 bar, H2/CO =1 and 350 °C), green = Fe2TiO5, red = Fe2O3 and blue = Fe3O4. After 7 hours under reaction conditions, green = Fe2TiO5, red = FeCx and blue = Fe3O4. (b3-b5) Fe K-edge spectra from 28 x 28 nm2 single pixels from three different regions. Experimental data noted by (•) and fitted data by (), respectively. Figure 2](/content/sites/default/files/images/science/highlights/2012/hayter_fto_fig2.jpg)
The material under study is the Ruhrchemie catalyst, selected as an important model system applied in the Fischer-Tropsch industry. It consists of a bulk iron oxide promoted with titanium, zinc and potassium oxides. The elemental distribution of all the components within the fresh catalyst was obtained by 3D nano-tomography of one of the catalytic particles (Figure 2(a)) above and below the X-ray absorption K-edges of Fe and Zn, followed by background subtraction, image averaging and magnification correction and subtraction [2] to map the main chemical elements in the catalyst at tens of nm resolution. It is interesting to note that the Zn particles are mainly associated with Ti in the catalyst rather than with Fe, which is inconsistent with the assumed role of ZnO as only a structural promoter. The catalyst chemistry was also analyzed by recording image stacks at the iron K-edge, first at room temperature in a helium atmosphere and then under FTO conditions over a period of 7 hours. Figure 2(b) shows selected 2D chemical maps of the particle obtained from linear combination least square fits of single pixel (28 nm) XANES to reference compounds [1], verified using principal components analysis. By studying the evolution of the species during reaction, it was noted that Fe2O3 was reduced before Fe2TiO5, which was still present after 5 hours of reaction. After 7 hours of reaction, the iron phase was fully reduced to catalytically active species: Fe3O4 and iron carbides.
By following single-pixel chemistry of a working catalyst at nm-scale resolution, we have correlated catalyst reaction chemistry with specific chemical forms within the starting particle. We found that, of the main starting materials within the fresh catalyst, Fe2O3 was best reduced to active forms of Fe for FTO synthesis. Fe2TiO5, which is formed as a result of calcining with TiO2, was found to be not as chemically active. This type of spatially resolved chemical information can inform synthesis protocols for FTO and other catalysts for optimized activity.
With the present results we have demonstrated that recent advances in TXM combined with our specially designed operando spectroscopy reactor, make it possible to study dynamic changes of a single catalyst particle under high pressure (10-30 bar) and temperature (350-500 οC).
Rock on Fire from SSRL on Vimeo.
The authors acknowledge financial support from The Dow Chemical Company. The Stanford Synchrotron Radiation Lightsource (SSRL) is supported by the US Department of Energy, Office of Basic Energy Sciences. We wish to thank to A. van der Eerden (Utrecht University) and D. Van Campen (SSRL) for their help with the design of the reactor cell. We further acknowledge P. Williams and T. Kao (SSRL) for their help during data processing and R. Marks (SSRL) for setting up the gas flow system.
1) F. Meirer et al. (2011) J. Synchr. Radiat. 18:773-781.
2) Y. Liu et al. (2012) Anal. Bioanal. Chem. 404:1297-1301.
I. Gonzalez-Jimenez, K. Cats, T. Davidian, M. Ruitenbeek, F. Meirer, Y. Liu, J. Nelson, J.C. Andrews, P. Pianetta, F.M.F. de Groot, B.M. Weckhuysen (2012) Hard X-Ray Nanotomography of Catalytic Solids at Work, Angew. Chem. Int. Ed. v51. (doi: 10.1002/ anie.201204930).

