Energy storage materials, such as batteries, are of increasing importance in the modern world. They support the storage and distribution of electricity generated by different mechanisms, enabling the use of green power sources when the resource itself is unavailable (for example, solar energy at night or wind energy on a calm day). Such devices also provide energy portability for consumer electronics and zero-emission options for transportation, in either hybrid- or fully-electric vehicles. As dictated by the Kyoto Protocol, in the interest of maintaining a safe and healthy planet for future generations, emissions from both transportation and industrial energy production must be reduced over the coming years. Clearly energy storage materials will play a critical role in realizing this goal.
A good understanding of existing technologies often points the way toward the manufacture of better devices; knowledge of their operational and failure modes can lead to ways to characterize and correct these problems in future products. While many impressive battery technologies exist today, the understanding of their operation is somewhat limited, which makes it very challenging to improve their performance. Battery microstructure – specifically, the assembly of particles into electrode systems – plays a critical role in determining the efficacy of the device, but available inspection technologies have limited characterization efforts to cells that have been depackaged. Electron microscopy, for example, is a common approach to battery electrode metrology, but requires a specialized sample preparation scheme that may not deliver results representative of the real cell. These preparations also present a practical limit to understanding how the battery works under realistic conditions as well as prohibiting real-world studies of changes in microstructure – the very mechanisms that may lead to failure – as a function of operation.
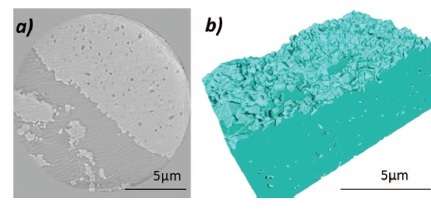
Researchers at SSRL, General Motors, Imperial College London, National Taiwan University, and elsewhere have recently begun experimenting with 3-D transmission X-ray microscopy (TXM), in order to gain new insight into the microstructure of battery electrodes. However, an open question that remains is one of length scale – what information is obtained depending on the spatial resolution and 3-D field-of-view in a TXM investigation.
A recent paper jointly authored by University College London, Imperial College London, the University of Manchester, and Xradia, Inc., documented a comprehensive multi-length scale investigation which was performed in order to better address this question and enable the introduction of guidelines for future battery microstructure investigations. In this paper (Shearing et. al., "Multi Length Scale Microstructural Investigations of a Commercially Available Li-Ion Battery Electrode", Journal of The Electrochemical Society, 159 (7) A1023-A1027 (2012)), laboratory micron-scale imaging was extended to both laboratory and synchrotron nano-imaging in order to characterize the types of information obtained at each length scale. The study found that laboratory micro-imaging reveals the bulk pore and transport pathways, while laboratory nano-scale TXM delivers the precise pore pathways (for example, tortuosity) and particle shapes. Utilizing synchrotron nano-scale TXM, performed at SSRL Beam Line 6-2, intra-particle imperfections were clearly revealed, which have only been detected in the past on specially-prepared samples imaged with TEM.
Using the power of both laboratory- and synchrotron-TXM, the precise 3-D information about battery micro- and nano-structures will enable a new generation of electrochemical research. The flexibility of the non-destructive approach is already enabling novel functional studies of electrode operation, and the recent work at SSRL will help guide this research to optimized results.
P. R. Shearing, N. P. Brandon, J. Gelb, R. Bradley, P. J. Withers, A. J. Marquis, S. Cooper and S. J. Harris, "Multi Length Scale Microstructural Investigations of a Commercially Available Li-Ion Battery Electrode", Journal of The Electrochemical Society, 159 (7) A1023-A1027 (2012) [DOI: 10.1149/2.053207jes]

