An important building block of a new sustainable energy infrastructure is the transition of the transportation sector from inefficient internal combustion engines towards electric drives. Proton exchange membrane fuel cells (PEMFCs) are promising power sources since they can generate electricity from the electrochemical oxidation of a fuel, e.g. hydrogen or methanol, which would provide higher energy storage density than lithium batteries and compatibility with the existing hydrocarbon fuel distribution network.
Large-scale deployment of fuel cells, however, has been hampered by cost and performance issues with catalyst materials needed to spur the oxidation of fuel and the reduction of oxygen, which occur in separate anode and cathode compartments of the fuel cell, respectively. In particular the oxygen reduction reaction (ORR), even when using the most active catalyst material, platinum, suffers from significant loss of efficiency and, moreover, degradation of the catalyst under long-term operating conditions.
A precise understanding of the platinum surface chemistry underlying the slow ORR mechanism and catalyst degradation has been very limited due to experimental difficulties. Spectroscopic techniques can probe chemical bonding, but since penetration depth and probing depth cannot be separated, much needed studies of the Ångström-thin interface region where the reaction takes place could not be realized under in situ conditions resembling a working fuel cell, where the interface is buried under a thick electrolyte layer.
A team of researchers led by SSRL Research Associate Daniel Friebel has addressed this problem and developed a new, more powerful way to probe a platinum catalyst in an electrochemical environment with x-ray absorption spectroscopy (XAS). Their new technique and the in situ observation of the formation of surface platinum oxide are described in a paper appearing in Physical Chemistry Chemical Physics, a publication of the Royal Chemical Society.
Previous XAS experiments generally employed platinum nanoparticles. Despite their small size, they still have an unfavorably high ratio of bulk vs. surface atoms where contributions from the former dominate the x-ray absorption spectra. Furthermore, typical nanoparticle samples have a broad distribution of sizes and shapes, making the data interpretation even harder. In order to eliminate bulk contributions to Pt L3 x-ray absorption spectra, the researchers prepared an ultrathin model catalyst, consisting of one Pt monolayer grown on a Rh(111) single-crystal surface. In addition to the enhancement of surface sensitivity, they made use of the high energy resolution fluorescence detection (HERFD) technique that partially eliminates the core-hole lifetime broadening effect in XAS, thus revealing significantly more detailed spectral features than conventional XAS.
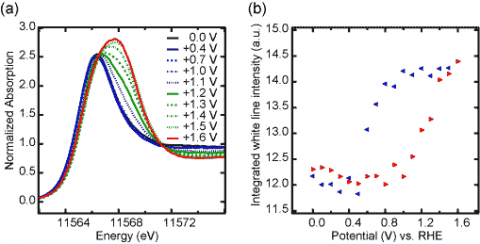
Hence, the researchers were able to establish unambiguous spectral fingerprints for surface Pt–O interactions ranging from chemisorbed oxygen atoms on the intact metallic surface to surface oxide formation, where metallic Pt–Pt interactions are absent. The surface oxide, once it was formed, was found very difficult to remove (Fig. 1), and could therefore play an important role in the poor performance of fuel cells.
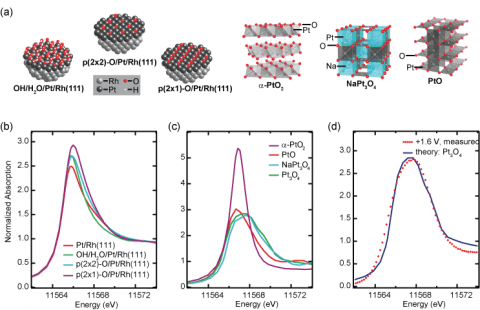
Ab initiomultiple-scattering calculations were employed to predict the shape of the white line, a characteristic absorption maximum due to excitation from Pt 2p into unoccupied 5d states, for model structures containing either chemisorbed oxygen or platinum oxide species (Fig. 2). Chemisorbed oxygen-containing species were found to induce only subtle peak intensity increases, while the overall spectral shape remains very similar to that of metallic Pt. Much more significant changes were found for Pt oxides, including a strong dependence of the white line width of the Pt oxidation state and coordination environment, which can be explained with ligand field splitting of the Pt 5d states. Pt oxide formation was experimentally confirmed with complementary in situ EXAFS measurements.
The weak spectral signature of chemisorbed O or OH might be much more difficult, if not impossible, to detect in a conventional XAS experiment, especially when bulk Pt contributes to the spectra. Hence, for previous observations of the increase and significant broadening of the white line at high potentials, the researchers suggest a reinterpretation in terms of oxide formation rather than OH adsorption. Moreover, this oxide signature has been observed already at significantly lower potentials for Pt nanoparticles than on the Pt/Rh(111) system studied here. This implies that the importance of surface oxide might have been underestimated in modeling the ORR mechanism with regards to the origin of the high overpotential, as well as understanding the degradation of fuel cell catalysts.
D. Friebel, D. J. Miller, C. P. O’Grady, T. Anniyev, J. Bargar, U. Bergmann, H. Ogasawara, K. T. Wikfeldt, L. G. M. Pettersson, A. Nilsson, Phys. Chem. Chem. Phys. 13, 262–266 (2011) DOI: 10.1039/C0CP01434F.