![Figure 1. Fundamental biochemical reduction/oxidation processes involving methionine (MetS) sidechains. Structures shown to indicate amino acid within a peptide chain. [O] indicates an oxidative source (e.g. H2O2) and MSR refers to methionine sulfoxide reductase. (Image: Pierre Kennepohl) Figure 1.](/content/sites/default/files/images/science/highlights/2009/mets_oxidation_fig1.jpg)
Numerous diseases, as well as aging itself, are linked to uncontrolled oxidative processes that lead to irreversible damage and ultimately death. Defining and understanding these processes is one of the keys that may yet lead us to stop and possibly even reverse oxidative damage and thus effectively prevent and/or treat a wide range conditions. Oxidative damage to proteins has been studied quite extensively over the last decade and it has become clear that certain amino acids are more susceptible to oxidation than others. Notably, methionine (MetS) is remarkably susceptible to oxygenation to its sulfoxide form (MetSO). Under certain circumstances, this process is used in signalling and other normal biological function; a family of enzymes known as methionine sulfoxide reductases (MSRs) can reduce MetSO back to MetS (Fig 1). However, if this dynamic oxidation/reduction cycle is disrupted, or if it occurs where MSRs are unavailable, a build up of MetSO could ensue with negative consequences. In addition, under even more aggressive conditions, MetSO can be further oxygenated to its sulfone (MetSO2), for which enzymatic reduction is not believed to be feasible. An example where MetS oxidation is believed to play an important role is in age-related cataracts where a-crystallin, the most abundant protein in the eye lens (Fig 2), aggregates and causes a marked decrease in the transparency of the lens - eventually leadings to blindness. These protein aggregates show signs of oxidative damage, especially oxidized forms of MetS. Long-term UV light exposure has shown to increase oxidation of
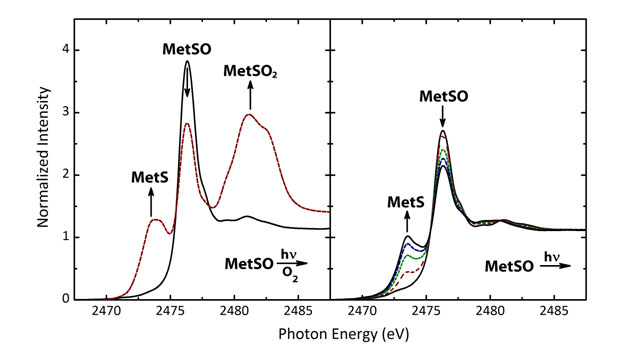
α-crystallin most notably at methionine residues. This basic information led Anusha Karunakaran-Datt and her doctoral advisor, Pierre Kennepohl at the University of British Columbia, to investigate the inherent photochemistry that is postulated to drive MetS oxidation in a-crystallin. Using sulfur K-edge X-ray absorption spectroscopy (XAS) to highlight redox changes occurring at the sulfur atom of methionine, they observed that visible-light photochemical processes can lead both to harmful oxidative processes (forming MetSO and MetSO2) as has generally been suspected, but also to photoreductive processes that allow MetSO to return to its native MetS form (Fig 3). As noted in their recent publication detailing their findings, the relative importance of the photooxidative and photoreductive processes is directly linked to the presence (or absence) of dioxygen (O2).
Under normal conditions, the crystalline lens is essentially anaerobic (free of O2), thus ensuring that oxidative damage can be reversed when our eyes our exposed to light. However, levels of O2 in the lens are believed to increase with age, allowing the negative oxidation processes to take over, thus creating the conditions that allow for formation of age-related cataracts. These studies therefore suggest that an important aspect of preventing age-related cataracts likely rests in exploring and controlling the permeability of the crystalline lens towards dioxygen. Current efforts are focused on exploring the detailed mechanisms of the observed photoreductive and photooxidative processes, as well as defining the energy profile of the observed photochemistry.
Further Readings
For more on the role of protein oxidation in age-related cataracts, see the following: (a) RJW Truscott, "Age-related nuclear cataract - oxidation is the key", Exp. Eye Res., 80, 709-725 (2005); (b) DL Williams, "Oxidation, antioxidants and cataract formation: a literature review", Vet. Ophthalmol., 9, 292-298 (2006); (c) MJ Davies and RJW Truscott, "Photo-oxidation of proteins and its role in cataractogenesis", J. Photochem. Photobiol. B, 63, 114-125 (2001); (d) L Takemoto and CM Sorensen, "Protein-protein interactions and lens transparency", Exp. Eye Res., 87, 496-501 (2008).
Details of experimental approach can be obtained from P Kennepohl, E Wasinger, and S DeBeer George, "X-ray spectroscopic approaches to the investigation and characterization of photochemical processes", J. Synch. Rad., accepted for publication.
A Karunakaran-Datt and P Kennepohl, "Redox Photochemistry of Methionine by Sulfur K-edge X-ray Absorption Spectroscopy: Potential Implications for Cataract Formation", J. Am. Chem. Soc., 131(10), 3577-3582 (2009).




