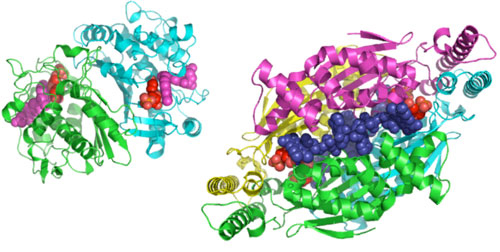
Classical thymidylate synthases, encoded by the thyA and TYMS genes, are present in most eukaryotes, including humans, and are frequently targeted by chemotherapeutic and antibiotic drugs. A recently discovered class of thymidylate synthases, the FDTSs encoded by the thyX gene has been found primarily in prokaryotes and viruses including several pathogens and biological warfare agents (see http://www.cdc.gov). Several organisms, including human pathogens, rely solely on thyX for thymidylate synthesis. FDTSs share no structure or sequence homology with classical thymidylate synthases (Fig 1), and thus present a promising new frontier for antibacterial/antiviral drug development.
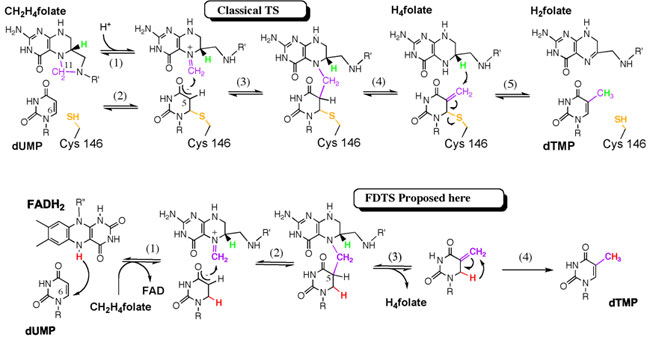
In an article published in Nature, research team lead by Prof. Kohen has unraveled an unusual mechanism for the FTDS catalysis. A significant component of the study involved structural data collected at SSRL Beam Line 9-2 by Dr. Mathews following his successful crystallization of the wild type and two critical mutants of FDTS. Other experimental components include kinetic and isotopic analysis of the enzyme and mutants. The novel mechanism is an example of thymidylate biosynthesis that occurs without an enzymatic nucleophile (Scheme 1). The findings indicate that the putative active site nucleophile is not required for FDTS catalysis, and no alternative nucleophilic residues capable of serving this function can be identified. This study suggests that a hydride is transferred from the reduced flavin cofactor directly to the uracil ring, followed by an isomerization of the intermediate to form the product thymidylate as illustrated in Scheme 1). The observations indicate a very different chemical cascade than that of classical thymidylate synthases or any other known biological methylation. The findings and chemical mechanism proposed here, together with available structural data, suggest that selective inhibition of FDTSs, with little effect on human thymine biosynthesis, should be feasible. Because several human pathogens depend on FDTS for DNA biosynthesis, its unique mechanism makes it an attractive target for antibiotic drugs.
Myllykallio, H. et al. An alternative flavin-dependent mechanism of thymidylate synthesis. Science 297, 105-107 (2002).
Agrawal, N., Lesley, S. A., Kuhn, P. & Kohen, A. Mechanistic studies of a flavin-dependent thymidylate synthase. Biochemistry 43, 10295-10301 (2004).
Mathews, I. I. et al. Functional analysis of substrate and cofactor complex structures of a thymidylate synthase-complementing protein. Structure 11, 677-690 (2003).
Eric M. Koehn, Todd Fleischmann, John A. Conrad, Bruce A. Palfey, Scott A. Lesley, Irimpan I. Mathews, and Amnon Kohen, "A Novel Chemical Mechanism of Thymidylate Biosynthesis in Human Pathogens Containing the thyX Gene" Nature 458, 919 (2009).

